| Class | 10th |
| Subject | Science (NCERT) |
| Category | Important Questions |
Carbon and its Compound Class 10 Science Chapter 4 Important Question Answer
Q1. Explain the following processes with help of chemical reactions: Most Important
(a) Esterification
(b) Saponification
(c) Hydrogenation
Ans – (i) Esterification reaction: Esters are most commonly formed by reaction of an acid and an alcohol. Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give an ester.
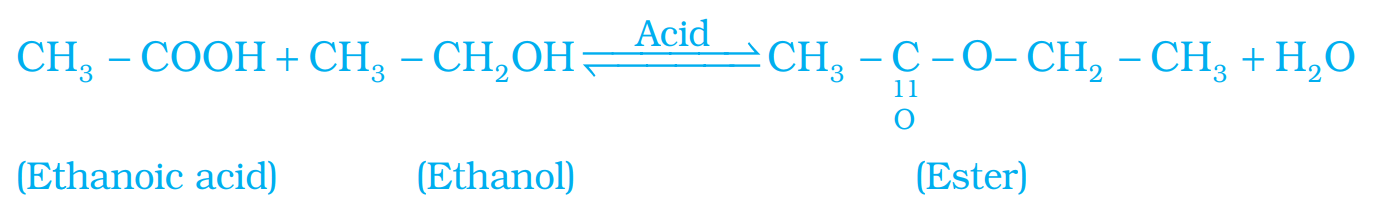
Generally esters are sweet smelling used in making perfumes and as flavouring agents.
(ii) Saponification : On treating with sodium hydroxide, which is an alkali, the ester is converted back to alcohol and sodium salt of carboxylic acid. This reaction is known as saponification.
CH3COOC2H5 C2H5OH + CH3COONa
(iii) Hydrogenation : Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons. This reaction is commonly used in the hydrogenation of vegetable oils.
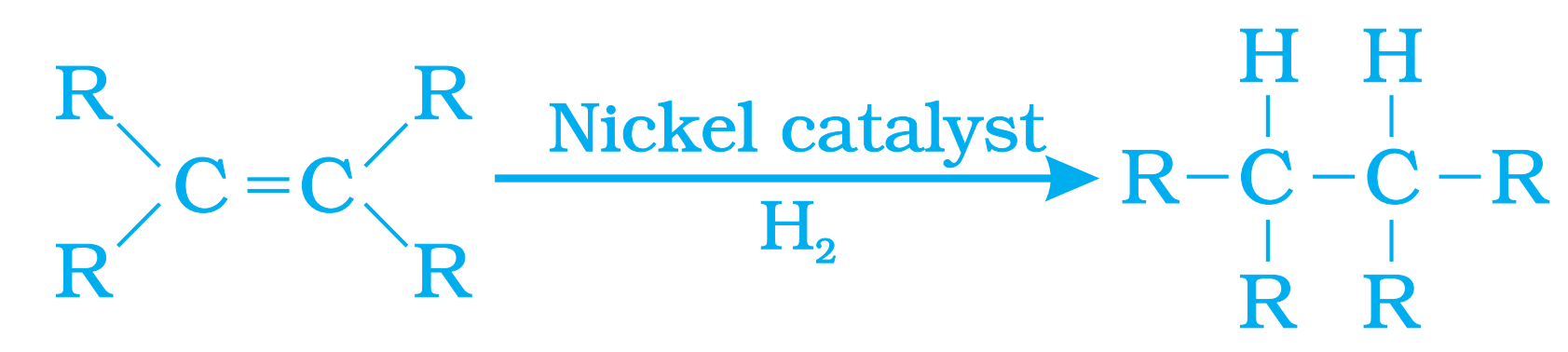
Q2. Explain in detail the Nomenclature of carbon compounds. Most Important
Ans – Nomenclature of carbon compounds can be done by the following method –
(i) Identify the number of carbon atoms in the compound. For example compound having one carbon uses prefix “meth”. A table for such prefix is given as :
| Number of Carbon atoms | Prefix |
1 2 3 4 5 6 7 8 9 10 | Meth Eth Prop But Pent Hex Hept Oct Non Dec |
(ii) In case a functional group is present, it is indicated in the name of the compounds with either a prefix or a suffix .
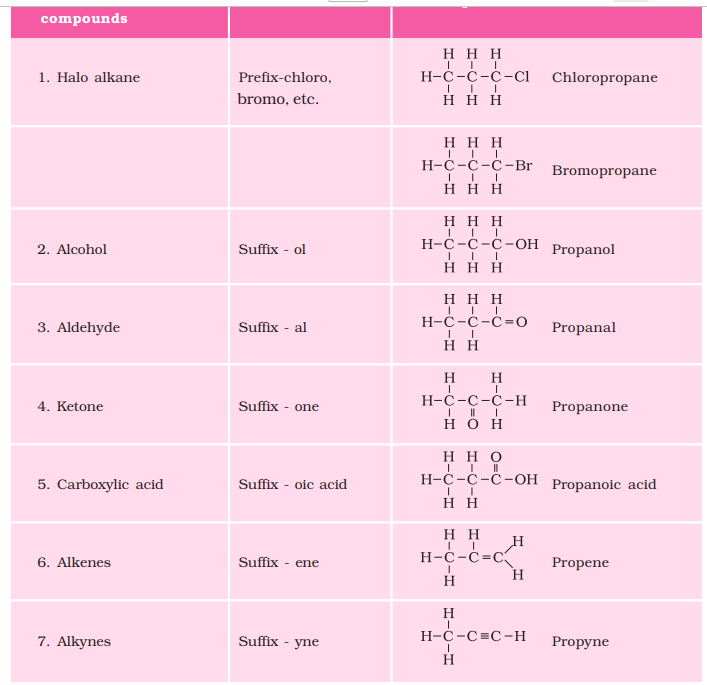
(iii) If the name of the functional group is to be given as a suffix, and the suffix of the functional group begins with a vowel a, e, i, o, u, then the name of the carbon chain is modified by deleting the final ‘e’ and adding the appropriate suffix. For example, a three-carbon chain with a ketone group would be named in the following manner – Propane – ‘e’ = propan + ‘one’ = propanone.
(iv) Carbon compounds with single bond use ‘ane’ as suffix, whereas carbon compounds with double bond use ‘ene’ and with triple bond use ‘yne’.
Formulas : Alkanes (CnH2n+2) , Alkenes (CnH2n), Alkynes (CnH2n-2).
Q3. What happens when ethanol reacts with the following: Most Important
(a) Acidified potassium dichromate
(b) Sodium
(c) Hot. Conc. H2SO4
Ans –
(a)

Alkaline potassium permanganate or acidified potassium dichromate are oxidising alcohols to acids, that is, adding oxygen to the starting material.
(b) 2Na + 2CH3CH2OH → 2CH3CH2O–Na+ + H2
When ethanol react with sodium then sodium ethoxide and hydrogen gas is the product.
(c)

When ethanol react with hot concentrated sulphuric acid than sulphuric acid can be regarded as dehydrating agent which removes water from ethanol.
Q4. Detergents are effective in Hard Water also. Comment.
Ans – Detergents are generally sodium salts of sulphonic acids or ammonium salts with chlorides or bromides ions, etc. Both have long hydrocarbon chain. The charged ends of these compounds do not form insoluble precipitates with the calcium and magnesium ions in hard water. Thus, they remain effective in hard water.
Q5. Explain the mechanism of cleaning action of soaps. Most Important
Ans – The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. The dust particles are then easily rinsed away by water.
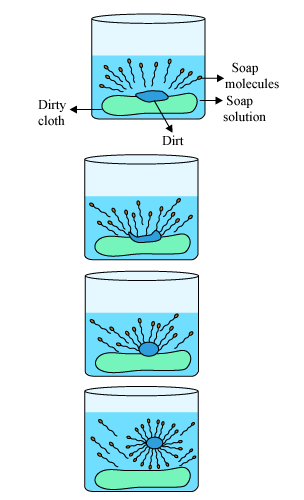
Q6. Give two differences between soaps and detergents.
Ans.
| Soaps | Detergents |
| Molecules of soap are sodium or potassium salts of long-chain carboxylic acids. | Molecules of detergents are sodium salts of sulphonic acids or ammonium salts with chlorides and bromides ions. |
| Soaps are useful in soft water only. | Detergents are useful in both soft water and hard water. |
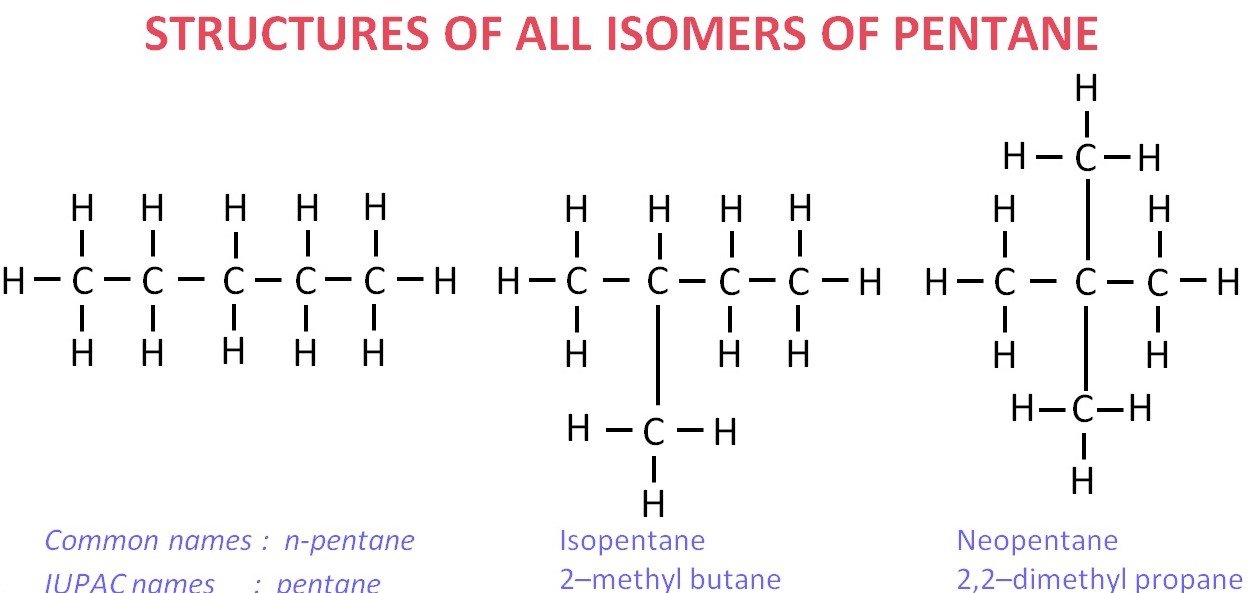
Q7. Draw the structures of all possible isomers of pentane. Most Important
OR
Draw all the structural isomers of pentane (C5H12).
Ans –
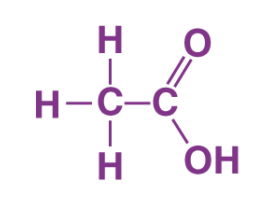
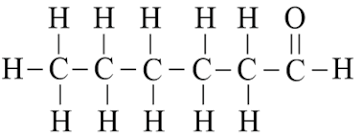
Q8. Draw the structures for the following compounds:
(a) Ethanoic acid
(b) Hexanal
(c) Butanone
(d) Butane
Ans –
(a)
(b)
(c)
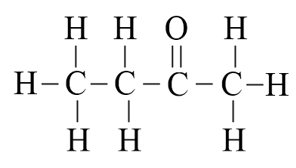
(d)
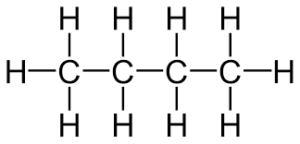
Q9. What is the difference between two consecutive homologous ?
(a) In terms of molecular mass
(b) Number of carbon and hydrogen atoms
(c) Chemical properties
Ans – (a) 14 unit
(b) CH2 unit
(c) Chemical properties remain similar in a homologous series.
Q10. How would you distinguish experimentally between an alcohol and carboxylic acid ?
Ans – Therefore, Carboxylic acid and Alcohol can be distinguished by using Sodium bicarbonate as, Carboxylic acid produces a brisk effervesce when reacted with it, whereas Alcohols do not produce anything when reacted with Sodium bicarbonate.
Q11. Write two uses of Ethanol.
Ans – (i) Ethanol is used as a solvent in paints, gums, resins etc.
(ii) It is used in the manufacture of alcoholic beverages.
Q12. Explain with examples the following properties of carbon compounds: Most Important
(a) Oxidation Reaction
(b) Addition Reaction
(c) Substitution Reaction
Ans –
(a) Carbon compounds can be easily oxidised on combustion. In addition to this complete oxidation, we have reactions in which alcohols are converted to carboxylic acids –

(b) Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons. This reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst. Vegetable oils generally have long unsaturated carbon chains while animal fats have saturated carbon chains.
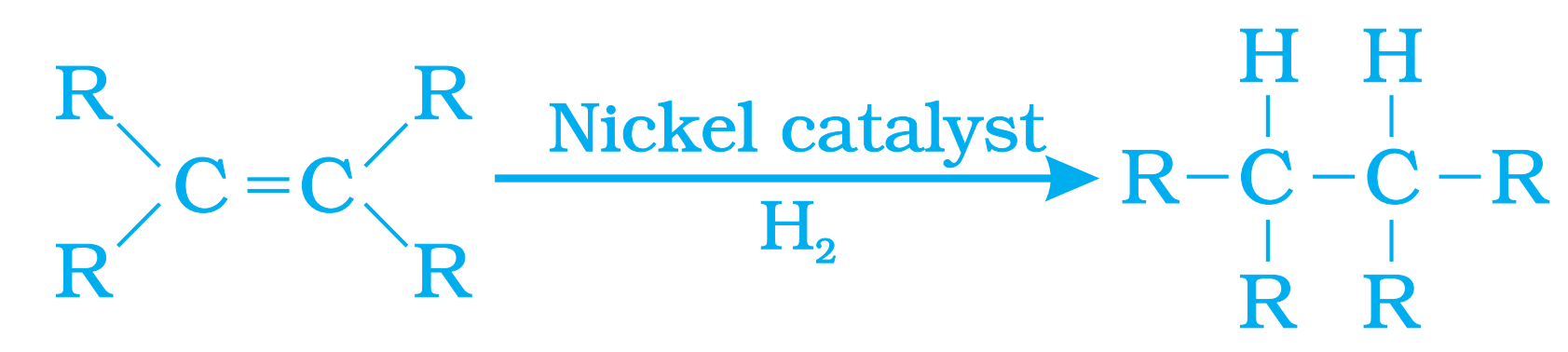
(c) Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen atoms one by one. It is called a substitution reaction because one type of atom or a group of atoms takes the place of another. A number of products are usually formed with the higher homologues of alkanes.
CH4 + Cl2 → CH3Cl + HCl (in the presence of sunlight)
Q13. What do you mean by Oxidising agent? Why is conversion of ethanol to ethanoic acid an oxidation reaction? Give chemical reaction also.
Ans – Some substances are capable of adding oxygen to others. These substances are known as oxidising agents. Alkaline potassium permanganate or acidified potassium dichromate are oxidising alcohols to acids, that is, adding oxygen to the starting material. Hence they are known as oxidising agents.
During the Conversion of ethanol to ethanoic acid, reaction occur in the presence of oxidising agent such as Alkaline potassium permanganate or acidified potassium dichromate. Carbon compounds can be easily oxidised on combustion. So this reaction is an oxidation reaction.

Q14. Alcohol is a clean fuel. Comment.
Ans – Alcohol is a clean fuel as it gives rise to only carbon dioxide and water on burning in sufficient air (oxygen). It doesn’t pollute environment.
Q15. Name the functional group present in Butanone.
Ans – ketone
Q16. Give chemical equation for the reaction of ethanoic acid with following: Most Important
(a) NaOH
(b) Na2CO3
(c) NaHCO3
(d) CH3CH2OH in the presence of acid
Ans –
(a) CH3COOH + NaOH → CH3COONa + H2O
(b) 2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
(c) CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
(d) 
Q17. Write the names of the following compounds : Most Important
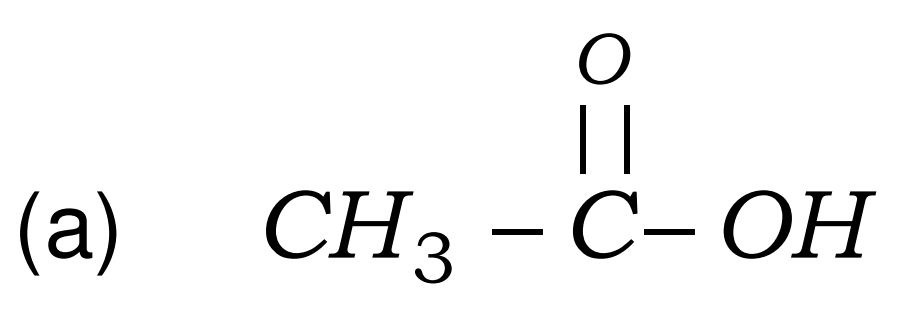
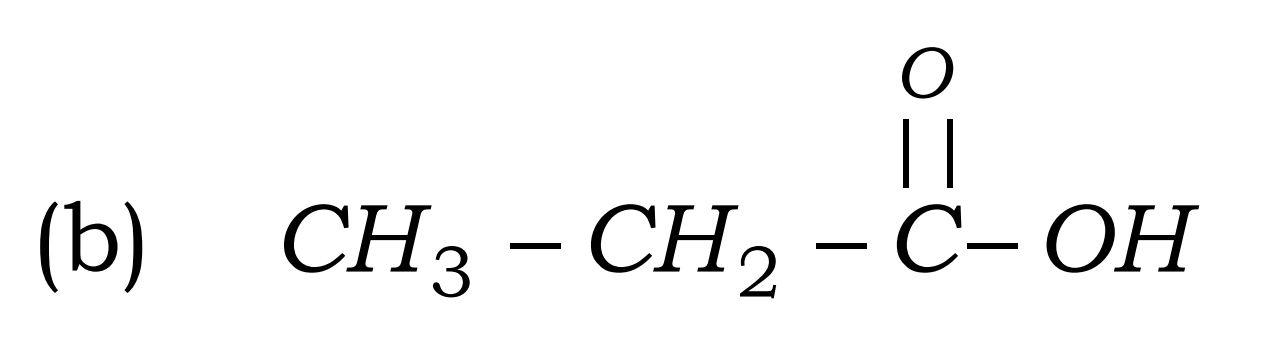
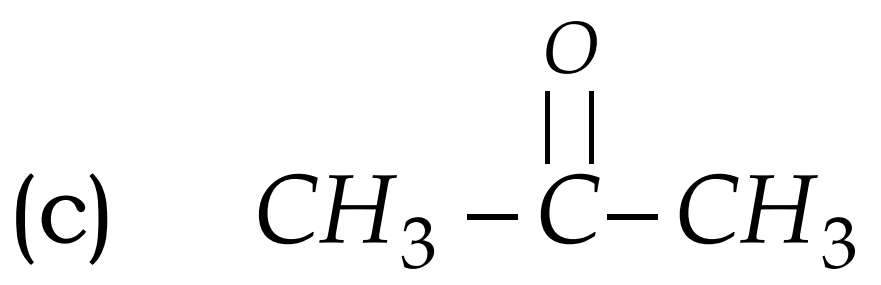
- CH3-CL
- CH3-CH2-CH2-OH
- CH3 – CH2 – Cl
- CH3 – CH2 – OH
- CH3 – CH2 -CHO
- CH3-CH2-CH2– Br
- CH3-C≡CH
- CH3-OH
- CH3-CH=CH2
- CH3-CH2-Br
- CH3COOH
- CH3Br
- CH3COCH3
- CH3CHO
- CH3-CH2-COOH
- CH3-CH2-CH2-CH3
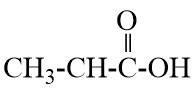
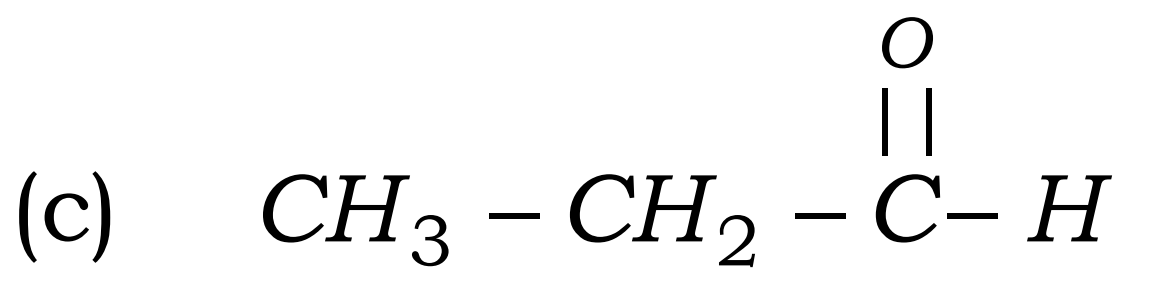
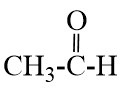
Ans –
- Ethanoic acid
- Propanoic acid
- Ethyl acetate
- methyl chloride
- propan-1-ol
- chloroethane
- ethanol
- propanal
- 1-bromobutane
- propyne
- methanol
- propene
- bromoethane
- ethanoic acid
- methyl bromide
- acetone
- acetaldehyde
- propanoic acid
- butane
- propionic acid
- butanal
- acetaldehyde
Q18. What is hydrogenation? What is its industrial application?
Ans – Conversion of unsaturated hydrocarbon into saturated hydrocarbon by the addition of hydrogen in the presence of Nickel or palladium as catalyst is known as hydrogentation.
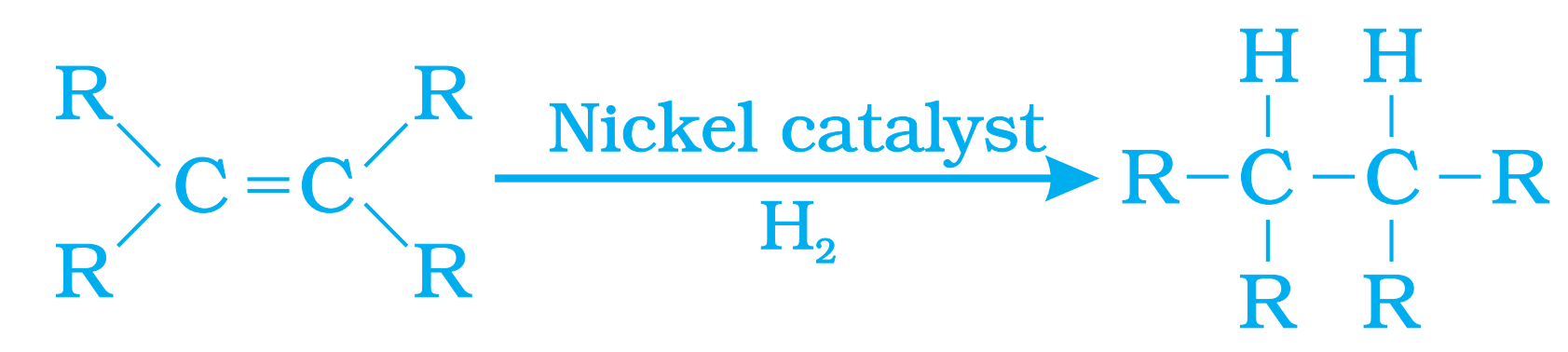
Industrial Application –
This process is used for conversion of vegetable oil into vegetable ghee.
vegetable oil + H2 Vegetable Ghee
Vegetable oils have double carbonic bond. When hydrogen gas is made to pass through these in the presence of Nickel action as catalyst at 473k, it converts into solid fat.
Q19. What is saponification reaction? Give example.
Ans – Saponification : On treating with sodium hydroxide, which is an alkali, the ester is converted back to alcohol and sodium salt of carboxylic acid. This reaction is known as saponification.
CH3COOC2H5 C2H5OH + CH3COONa
Q20. Which of the following hydrocarbons undergo addition reaction?
C2H6, C3H8, C3H6, C2H2
Ans – Only unsaturated hydrocarbon undergo addition reaction. So C3H8 and C2H2 will undergo addition reaction.
Q21. Define homologous series and functional group. Most Important
Ans –
Homologous series – Such a series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series.
Functional Group – The element replacing hydrogen is referred to as a heteroatom. These heteroatoms and the group containing these confer specific properties to the compound, regardless of the length and nature of the carbon chain and hence are called functional groups.
Q22. What are saturated and unsaturated hydro-carbons? Most Important
Ans –
- Saturated Hydrocarbons: Those hydrocarbons which contain only single bonds between carbon atoms. Examples include alkanes.
- Unsaturated Hydrocarbons: Those hydrocarbons which contain at least one double or triple bond between carbon atoms. Examples include alkenes and alkynes.
Q23. Explain two properties of carbon atom which lead to the huge number of carbon compounds.
Ans –
- Catenation – The carbon atoms have an astonishing property to combine and form bond with other carbon atoms to form long chain compounds. This property is known as catenation. In this, either long chain of carbon are in ring form or the carbon atoms join in single, double or triple bond.
- Tetravalency – Carbon has four electrons in the outermost shell. This is why its valency is four and it has got capacity to make bonds with other elements. Oxygen, Hydrogen, Nitrogen, Sulphur, Chlorine and many other elements can make new compounds with the help of carbon.
Q24. Write the formula of benzene and draw its structure. Most Important
Ans – Formula of benzene – C6H6
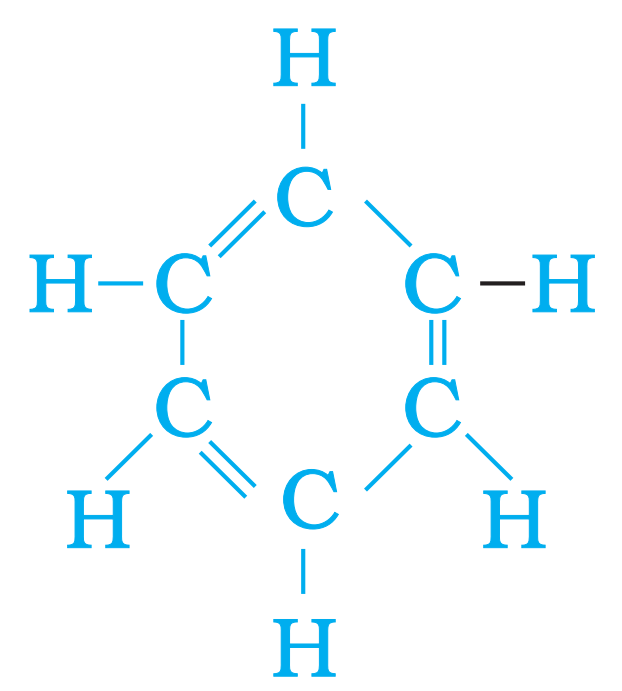
Q25. Differentiate between Hard water and Soft water.
Ans –
| Characteristic | Hard Water | Soft Water |
|---|---|---|
| Mineral Content | High concentration of calcium and magnesium ions | Low concentration of calcium and magnesium ions |
| Scale Formation | Forms scale or deposits when heated | Does not form scale when heated |
| Lathering | Does not lather well with soap | Lathers well with soap |
| Effect on Appliances | Mineral deposits can accumulate, reducing efficiency and lifespan | Less likely to cause mineral buildup, promoting longevity and efficiency |
| Common Ions Present | Calcium (Ca²⁺), magnesium (Mg²⁺), often in the form of bicarbonates, sulfates, or chlorides | May contain higher concentrations of sodium (Na⁺) or potassium (K⁺), depending on the softening process used |
Q26. How and why ethanol is denaturated ?
Ans – Ethanol is an important industrial solvent. To prevent the misuse of ethanol produced for industrial use, it is made unfit for drinking by adding poisonous substances like methanol to it. Dyes are also added to colour the alcohol blue so that it can be identified easily. This is called denatured alcohol.
Q27. Why are carbon and its compound used as fuels for most applications ?
Ans – When carbon and its compounds are made to burn in the presence of excess of air or oxygen large amount of heat, energy and light are produced. They result in continuous combustion. They do not require large amount of heat energy. These do not produce smoke or poisonous gases. No residue is left after their burning and has high calorific value. These are convenient to use as a fuel and except coal these do not leave any residence.
Q28. Why is Ethanoic acid named as Glacial Acetic acid ?
Ans – The melting point of pure ethanoic acid is 290 K and hence it often freezes during winter in cold climates. This gave rise to its name glacial
acetic acid.
Q29. What is Vinegar chemically?
Ans – CH3COOH
Q30. Define the terms Hetero-atom and a suffix. Explain with examples.
Ans – In a hydrocarbon, one or more hydrogens can be replaced by other elements , such that the valency of carbon remains satisfied. In such compounds, the elements replacing hydrogen is referred as hetero-atom.
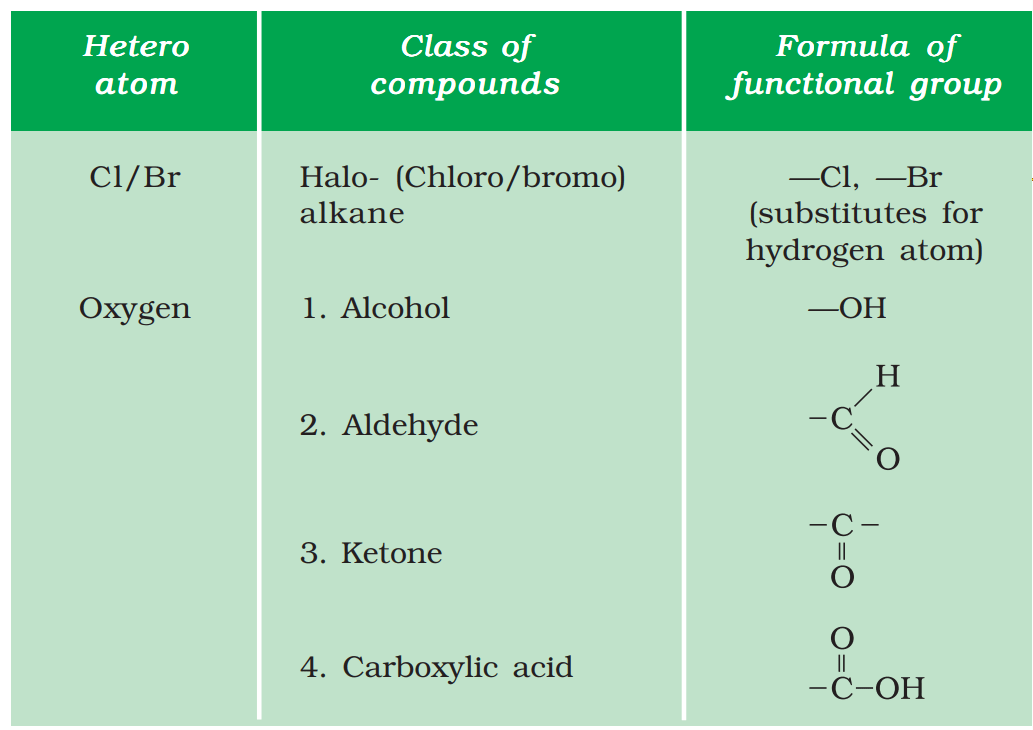
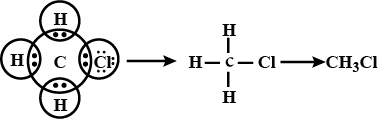
Q31. Explain the nature of covalent bond using the formation of bond in CH3Cl.
Ans –
Carbon has 4 valence electrons. In order to make octet, it shares each of the four electrons with each of the three hydrogen atoms and one chloride atom. Since, bonds are formed because of sharing of electrons, hence these are covalent bonds.
Q32. Draw structure of Cyclohexane.
Ans –
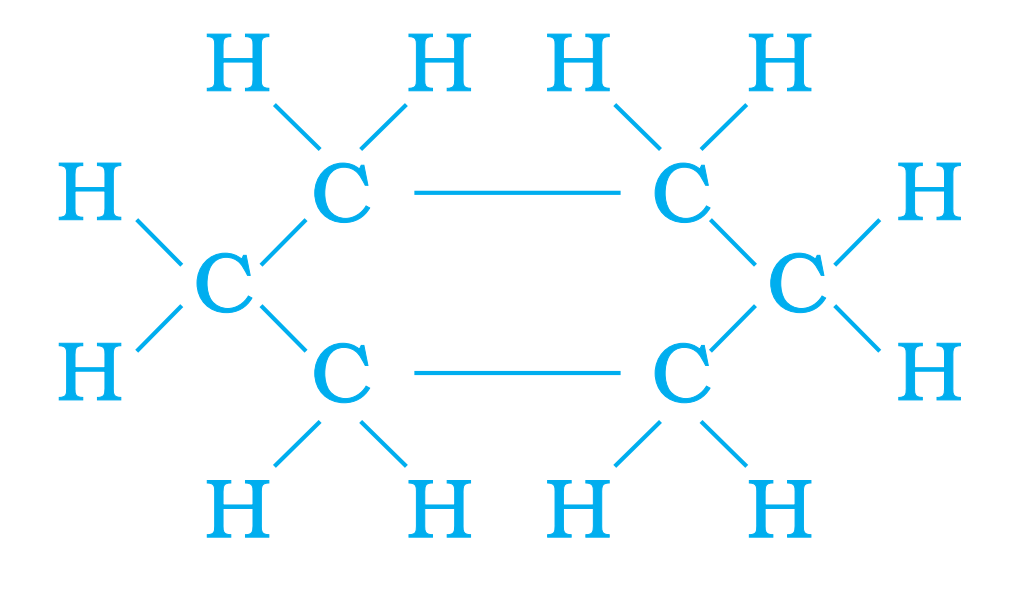
Q33. What do you mean by structural isomers ? Give one example.
Ans – The compounds with identical molecular formula but different structures are called structural isomers.
Example – Pentane have formula C5H12 can be represented as

Q34. Which is good for health a vegetable oil or Animal fat and why?
Ans – Vegetable oils are good for health because it contains unsaturated fatty acids which are used for cooking while animal fat contains saturated fatty acids which are said to be harmful for health.
Q35. Explain alkane, alkene and alkyne with suitable example.
Ans – Alkane : All these carbon compounds which contain only carbon and hydrogen are called hydrocarbons. Among these, the saturated hydrocarbons are called alkanes. Example – Methane, Ethane, Propane etc.
Alkene : The unsaturated hydrocarbons which contain one or more double bonds are called alkenes. Example – Ethene, Propene, Butene etc.
Alkyne : The unsaturated hydrocarbons which contain one or more triple bonds are called alkynes. Example – Ethyne, propyne, butyne etc.
Q36. Complete the following chemical reactions :
(i) CH3-CH2-OH + O2
(ii) CH3CH2OH
(iii) CH3COOC2H5
(iv) CH3COOH + NaHCO3
(v) Na + C2H5OH
(vi) C2H5OH
Ans –
(i) CH3-CH2-OH + O2 CH3-CO2-H(l) + H2O(l)
(ii) CH3CH2OH CH3COOH
(iii) CH3COOC2H5 CH3COONa+C2H5OH
(iv) CH3COOH + NaHCO3 CH3COONa+H2O+CO2
(v) 2Na + 2C2H5OH 2C2H5ONa+H2
(vi) C2H5OH C2H4 + H2O
Q37. Identify alkanes, alkenes and alkynes in following:
C2H2, C2H4, C3H8, C4H10, C4H8, C5H8
Ans – Alkanes (CnH2n+2) : C3H8 , C4H10
Alkenes (CnH2n) : C2H4 , C4H8
Alkynes (CnH2n-2) : C2H2 , C5H8
Q38. Write a short note on Micelles.
Ans – Clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle. Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the centre of the micelle.
| Class 10 Science NCERT Solution | Click Here |
| Class 10 Model Papers [Latest] | Download Papers |
| Class 10 Old Question Papers | Download Papers |