NCERT Solution of Class 10 Science Important MCQ Question Answer solution with pdf. Here We Provides Class 1 to 12 all Subjects NCERT Solution with Notes, Question Answer, CBSE and HBSE Important Questions, MCQ and old Question Papers for Students.
- Also Read :- Class 10 Science NCERT Solution
HBSE ( Haryana Board ) Solution of Class 10th Science all chapters important Question And Answer of Physics, Chemistry and Biology Important Question Solution.
Class 10 Science Important Question Answer for 2025 Chapter-Wise
HBSE Class 10 Science Chemistry Important Question Answer 2025
HBSE Class 10 Science Chapter 1 – Chemical Reaction and Equations Important Questions 2025
Q1. What do you mean by combination reactions? Give one example. Most Important
Q2. What do you mean by displacement reaction? Give one example. Most Important
HBSE Class 10 Science Chapter 2 – Acid, Bases and Salt Important Questions 2025
Q1. What is chlor-alkali process? Explain with the help of a chemical equation. Most Important
Q2. What is neutralisation reaction ? Give one example. Most Important
Q3. What is chemical formula of baking soda? Give chemical equation used in its preparation. Most Important
Q4. Four solution A, B, C and D when tested with a universal indicator, showed pH as 1, 7, 6 and 13 respectively Which solution is neutral, strongly alkaline, weak alkaline or weak acid and strongly acidic ? Most Important
HBSE Class 10 Science Chapter 3 – Metals and Non-Metals Important Questions 2025
Q1. Explain reactivity series with examples. Most Important
Q2. What are amphoteric oxides ? Give two examples of amphoteric oxides. Most Important
Q3. Write brief note on Electrolytic Refining. Most Important
Q4. In which state ionic compound exists? Why do they have high melting point ? Most Important
OR
Why do ionic compounds have high meltingpoints ?
Q5. What is thermit reaction? Give chemical equation for it. Most Important
HBSE Class 10 Science Chapter 4 – Carbon and its Compound Important Questions 2025
Q1. Explain the following processes with help of chemical reactions: Most Important
(a) Esterification
(b) Saponification
(c) Hydrogenation
Q2. Explain in detail the Nomenclature of carbon compounds. Most Important
Q3. What happens when ethanol reacts with the following: Most Important
(a) Acidified potassium dichromate
(b) Sodium
(c) Hot. Conc. H2SO4
Q4. Explain the mechanism of cleaning action of soaps. Most Important
Q5. Draw the structures of all possible isomers of pentane. Most Important
OR
Draw all the structural isomers of pentane (C5H12).
Q6. Explain with examples the following properties of carbon compounds: Most Important
(a) Oxidation Reaction
(b) Addition Reaction
(c) Substitution Reaction
Q7. Give chemical equation for the reaction of ethanoic acid with following: Most Important
(a) NaOH
(b) Na2CO3
(c) NaHCO3
(d) CH3CH2OH in the presence of acid
Q8. Write the names of the following compounds : Most Important
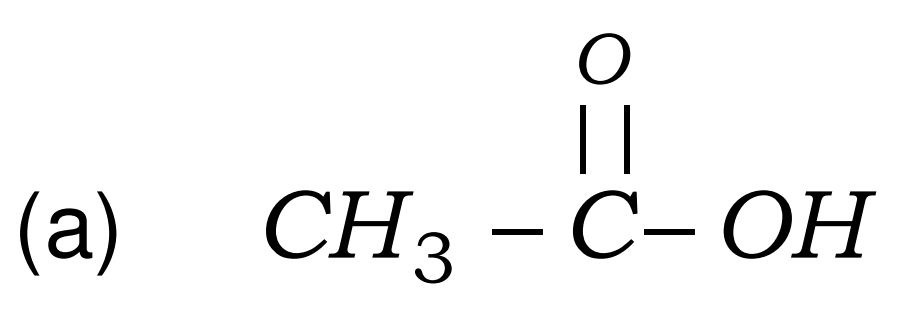
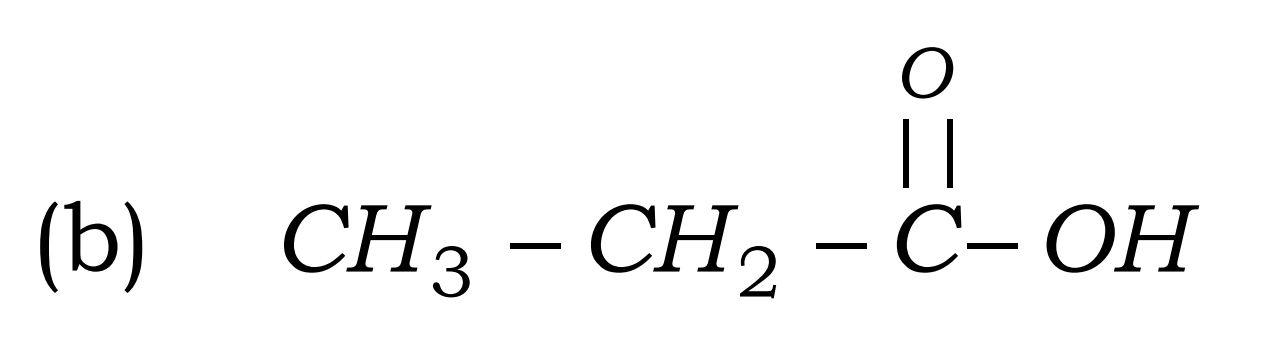
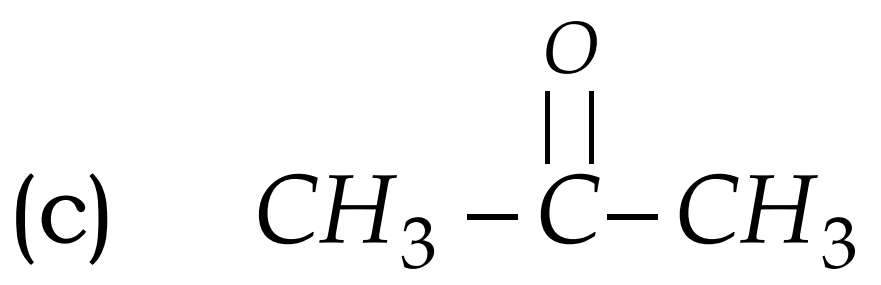
- CH3-CL
- CH3-CH2-CH2-OH
- CH3 – CH2 – Cl
- CH3 – CH2 – OH
- CH3 – CH2 -CHO
- CH3-CH2-CH2– Br
- CH3-C≡CH
- CH3-OH
- CH3-CH=CH2
- CH3-CH2-Br
- CH3COOH
- CH3Br
- CH3COCH3
- CH3CHO
- CH3-CH2-COOH
- CH3-CH2-CH2-CH3
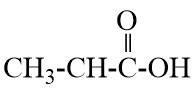
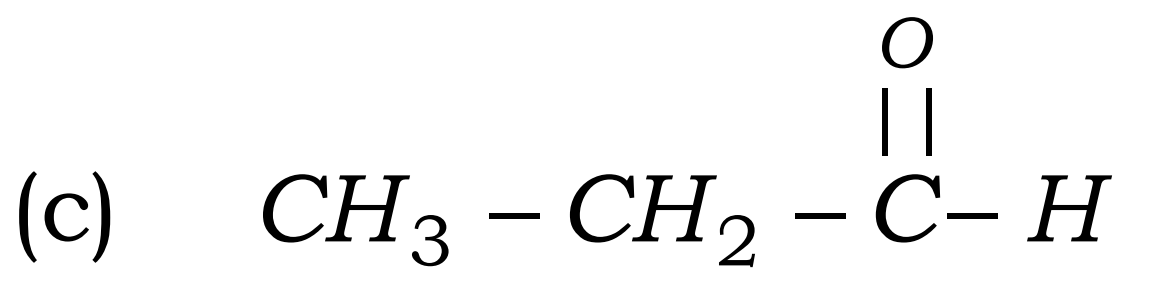
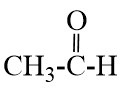
Q9. Define homologous series and functional group. Most Important
Q10. What are saturated and unsaturated hydro-carbons? Most Important
Q11. Write the formula of benzene and draw its structure. Most Important
Q12. Why are carbon and its compound used as fuels for most applications ? Most Important
HBSE Class 10 Science Biology Portion Important Questions 2025
HBSE Class 10 Science Chapter 5 – Life Processes Important Questions 2025
Q1. Describe the excretion in plants. Most Important
Q2. Describe the respiratory system of human beings. Most Important
OR
Explain the structure of human respiratory system with the help of well labelled diagram.
Q3. Describe the structure of nephron and how is urine produced? Most Important
OR
Describe structure and functioning of nephron. Most Important
Q4. Draw a well labelled diagram of excretory system and explain the process of urine formation in human being.
OR
Explain the structure of human excretory system with the help of a well labelled diagram.
Q5. What is lymph ? How is it transported ? What are its functions ? Most Important
Q6. Describe structure and functioning of human heart. Most Important
Q7. Draw a well labelled diagram of cross-section of leaf. Most Important
Q8. What are the differences between aerobic and anaerobic respiration? Most Important
Q9. What is photosynthesis? Write various events which take place during this process. Most Important
HBSE Class 10 Science Chapter 6 – Control and Coordination Important Questions 2025
Q1. What is geotropism ? Give an example. Most Important
Q2. Draw a well labelled diagram of a neuron. Most Important
Q3. What happens at synapse between two neurons? Most Important
Q4. Write down the functions of cerebellum and medulla. Most Important
Q5. Iodine is necessary for the synthesis of which hormone? Most Important
HBSE Class 10 Science Chapter 7 – How do Organisms Reproduce Important Questions 2025
Q1. Describe the process of budding in Hydra. Most Important
Q2. Draw a well labelled diagram of Longitudinal section of a flower. Most Important
Q3. Draw a well labelled diagram of human female reproductive system.
OR
Explain human female reproductive system with diagram.
Q4. What happens when the egg is not fertilized?
OR
Why does menstruation occur?
Q5. How is the process of pollination different from fertilization? Most Important
Q6. What are different contraceptive methods? How are they important for reproductive health? Most Important
HBSE Class 10 Science Chapter 8 – Heredity Important Questions 2025
Q1. How do Mendel’s experiment show that traits are inherited independently? Most Important
Q2. How is the sex of the child determined in human beings? Most Important
HBSE Class 10 Science Chapter 13 – Our Environment Important Questions 2025
Q1. Write full form of UNEP. Most Important
HBSE Class 10 Science Physics Important Questions 2025
HBSE Class 10 Science Chapter 9 – Light : Reflection and Refraction Important Questions 2025
This Table is Most important for this chapter. Must revise this.
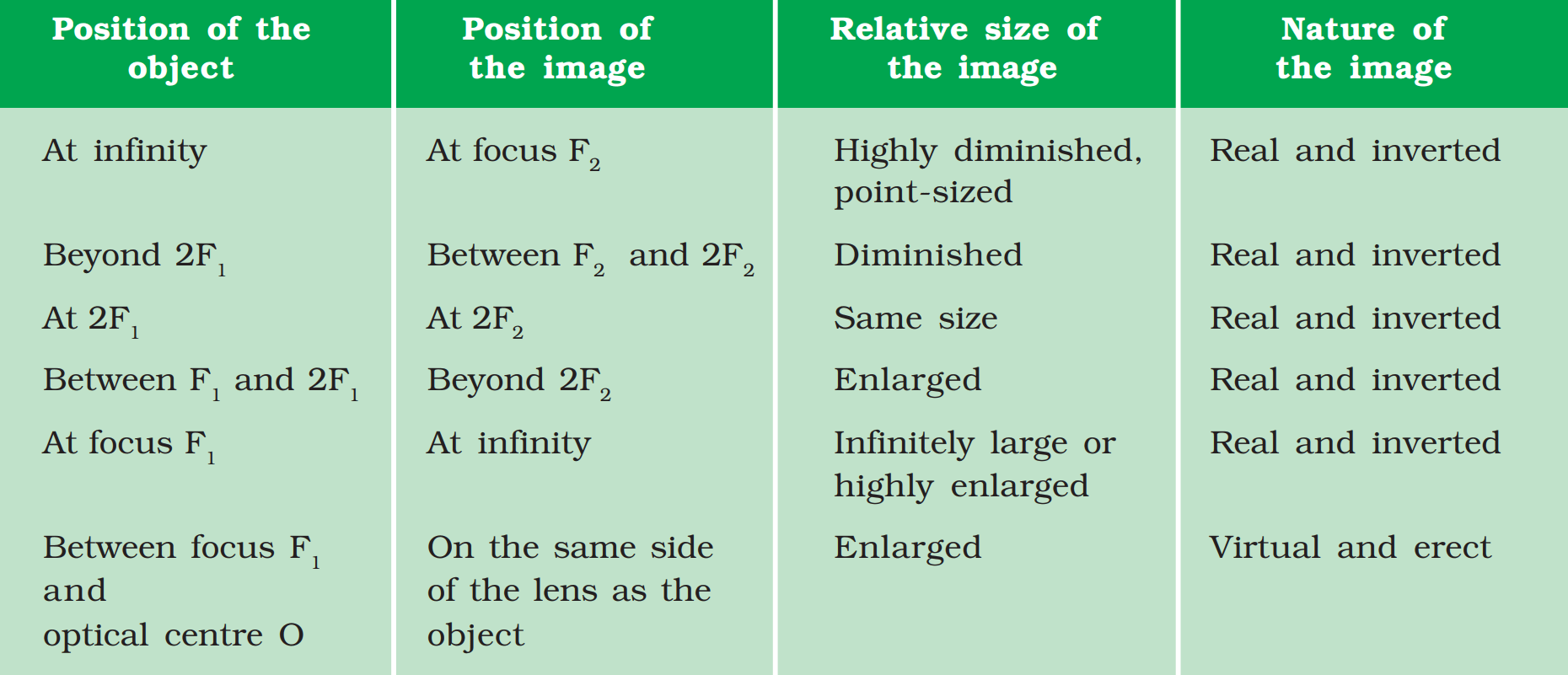
1. Nature, position and relative size of the image formed by a convex lens for various positions of the object
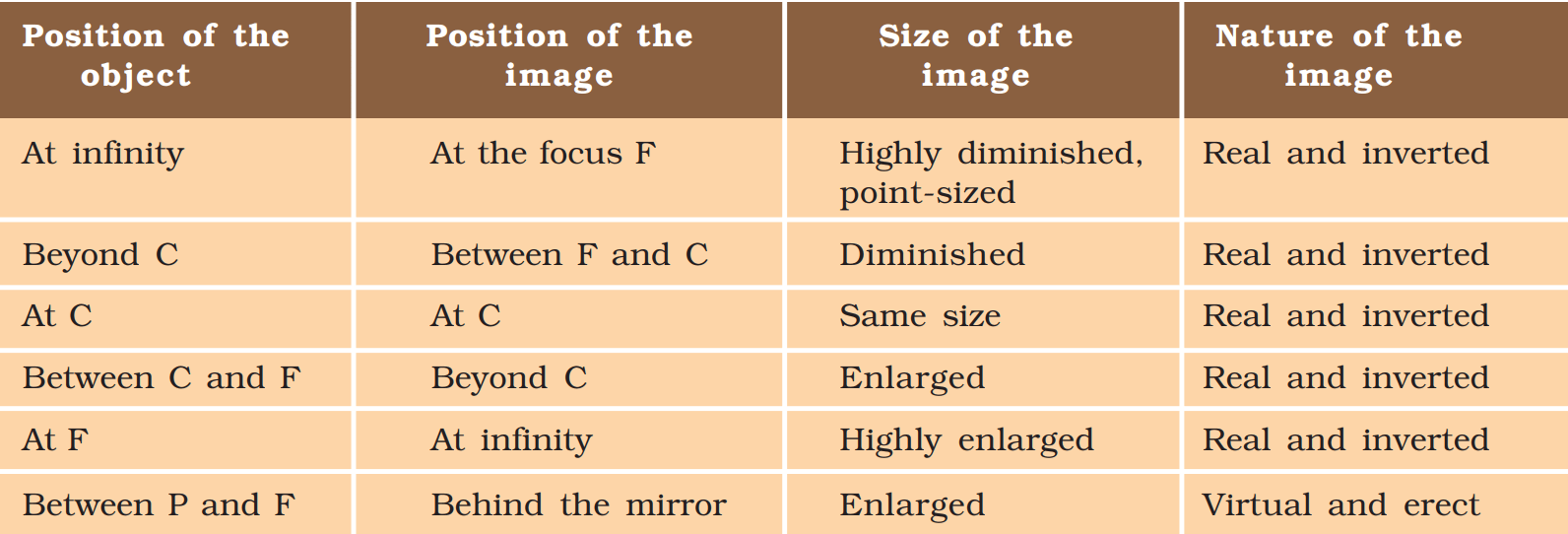
2. Image formation by a concave mirror for different positions of the object
Q1. What is power of a Lens ? Define one Dioptre (1D) power of a lens. Most Important
Q2. An object is placed at the centre of curvature (c) of a concave mirror. Draw the ray diagram to depict the position, size and the nature of image formed. Most Important
Q3. An object is placed at a position in between the main focus (F1) and the optical centre (O) of a convex lens. Draw a ray diagram showing the position, size and nature of the image formed. Most Important
Q4. Find the focal length of a lens of power -2.0 D. What type of lens is this? Most Important
Q5. Find the focal length of a lens of power +2.0 D. What type of lens in this ? Most Important
Q6. Find the focal length of a convex mirror whose radius of curvature is 32 cm. Most Important
Q7. What is refraction of light? Write the laws of refraction of of light. Most Important
Q8. Write the mirror formula. Most Important
HBSE Class 10 Science Chapter 10 – The Human Eye and The Colourful World Important Questions 2025
Q1. Explain why the planets do not twinkle. Most Important
Q2. The prism split the incident white light into a band of colours. Write these colours name in sequence.
OR
Write the name of colours in sequence found after splitting of white light passing through a glass prism.
HBSE Class 10 Science Chapter 11 – Electricity Important Questions 2025
Q1. Why are the coils of electric irons and toasters are made of an alloy rather than a pure metal ? Most Important
Q2. How can three resistors of resistances 2Ω, 3Ω and 6Ω be connected to give a total resistance of 1Ω ? Most Important
Q3. An electric refrigerator of power 400 W is allowed to run 10 hrs. per day. What is the cost of energy to operate it for 30 days at Rs. 4.00 per kWh? Most Important
Q4. What is resistance ? State its SI unit. On which factors does the resistance of a conductor depends ? Most Important
Q5. What do you mean by earthing? Why should electrical appliances be earthed ? Most Important
OR
What is the function of an earthwire? Why is it necessary to earth metallic appliances ?
HBSE Class 10 Science Chapter 12 – Magnetic Effect of Electric Current Important Questions 2025
Q1. Explain Right hand thumb rule. Most Important
Q2. What is a Solenoid? Draw the magnetic lines of force around a current carrying solenoid. Also throw some light on the use of solenoid. Most Important
Q3. Draw the magnetic lines of force around a bar magnet. Most Important
Q4. What do you mean by electromagnetic induction? Explain the use of Fleming’s right hand rule in finding the direction of current induced in the conductor. Most Important