Class 11 Chemistry BSEH Solution for Important Question Answer for Haryana board. CCL Chapter Provide Class 1th to 12th all Subjects Solution With Notes, Question Answer, Summary and Important Questions. Class 11 mcq, summary, Important Question Answer, Textual Question Answer in hindi medium and english medium are available of HBSE Board.
- Also Read – HBSE Class 11 All Subject Important Questions
HBSE Class 11 Chemistry Important Question with Answer for Haryana Board Solution.
HBSE Class 11 Chemistry Important Question Answer 2023
HBSE Class 11 Chemistry 2 Marks Important Questions with Answer 2023
Q1. Calculate the mass and charge of one mole of electrons.
Q2. Calculate mass percentage of different elements in Methane.
Q3. Calculate the frequency of yellow light of wavelength 750 nm.
Q4. Calculate the wavelength, frequency and wave- number of a light wave whose period is 2.0 x 10–10s.
Q5. Calculate the frequency (V) and wavenumber ( ) of blue light of wavelength 4800Å.
) of blue light of wavelength 4800Å.
Q6. Calculate frequency of radiation having wavelength 5800 Å.
Q7. Calculate pH of 0.01 M solution of sodium hydroxide.
Q8. The concentration of hydrogen ion in an acid is 4.8×10-3 M. What is its pH ?
Q9. Calculate the molarity of KOH in the solution prepared by dissolving its 5.6 g in water to form 250ml of solution.
Q10. Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g NaOH in water to form 250 mL of solution.
Q11. Calculate the molar mass of the following
(i) CO2
(ii) CH4
Q12. An atom of an element contains 29 electrons and 35 neutrons. Write the number of protons and the electronic configuration of the element.
Q13. What will be the minimum pressure required to compress 500 dm³ of air at 1 bar to 200 dm³ at 30°C.
Q14. Explain the physical significance of Vander Waals parameters.
Q15. Fluorine reacts with ice and results in the change :
H2O(s) + F2(g) →HF(g) + HOF(g)
Justify that this reaction is a redox reaction.
Q16. Write bond line formulas for: Isopropyl alcohol, Heptan-4-one
Q17. Determine the empirical formula of an oxide of iron, which has 69.9% iron and 30.1% dioxygen by mass.
Q18. Why is a solution of KOH used to absorb CO2 evolved during the estimation of carbon present in an organic compound?
Q19. Explain why is Na less reactive than K?
Q20. How do you account for the formation of ethane during chlorination of methane ?
Q21. Write chemical equations for combustion reactions of the following hydrocarbons:
(i) Hexyne
(ii) Toluene
Q22. Define surface tension. What is the effect of temperature on surface tension?
Q23. Define the following terms:
(i) Gibbs Energy
(ii) Enthalpy of Vaporisation.
Q24. What do you understand by the terms Ideal gas and Real gas ?
Q25. Give two uses of Plaster of Paris.
Q26. Explain common ion effect with an example.
Q27. Explain Inert Pair Effect.
Q28. What is Acid Rain? Give its consequence. Most Important
Q29. Write electronic configuration of :
(i) Cu (Atomic No. Cu = 29)
(ii) Fe2+ (Atomic No. Fe = 26)
Q30. Write IUPAC names of :
(i) CH3CH2CHCICH2OH
(ii) CH3CH2CN
Q31. Define Inductive effect and Electromeric effect. Most Important
Q32. Explain Ortho, Para, and Meta directing groups.
Q33. What are Lewis Acids and Lewis bases ? Give one example for each.
Q34. Discuss Avogadro’s Law.
Q35. Write two similarities between Lithium and Magnesium.
Q36. Explain Clark’s method to remove temporary hardness of water.
Q37. Complete the reactions:
(i) B2H6 + NH3 →
(ii) BF3 + LiH →
Q38. Write important applications of Silicons.
Q39. For equation H2(g) +I2(g) ⇌ 2HI(g) give the value of Kc and Kp.
Q40. Explain Hyperconjugation.
Q41. What is Huckel Rule ? Explain.
Q42. Draw shapes of dx2y2 and dxy orbitals.
Q43. Explain the reactivity order of Halogens ( F > Cl > Br > I).
Q44. The equilibrium constant for a reaction is 10. What will be the value of ΔG° = ? (R = 8.314 JK–1mol–1 , T = 300.0K) Most Important
Q45. How does H2O2 behave as bleaching agent ? Explain.
Q46. Is Boric acid a Protic acid ? Explain.
Q47. How can domestic waste be used as manure ?
Q48. What are the major causes of water pollution? Explain.
Q49. What is enthalpy of Combustion ? Give one example.
Q50. What is enthalpy of formation ? Give one example.
Q51. What will be the conjugate bases for the Bronsted acids HF and H2SO4 ?
Q52. What are Nucleophiles ? Give its types with examples.
Q53. What are Electrophiles? Give its types with examples.
Q54. What is Friedel-Crafts alkylation reaction? Most Important
Q55. What is Wurtz reaction? Most Important
Q56. What will be the wavelength of a ball of mass 0.1 kg moving with a velocity of 10ms–1 ?
Q57. What will be the conjugate acids for the Bronsted bases NH3 and HCOO– ?
HBSE Class 11 Chemistry 2½ Marks Important Questions with Answer 2023
Q1. Write the resonance structures for SO3 and NO2.
Q2. What do you know about hydrogen-bond?
Q3. Describe the hybridization in PCl5. Why are the axial bonds longer as compared to equatorial bonds?
Q4. Write differences between σ and π bonds.
Q5. Calculate the entropy change surroundings’, when 1.0mol of H2O(l) is formed under standard conditions:
ΔfH0 = – 286 KJ mol–1
Q6. What do you mean by lonic product of water (Kw) ?
Q7. Calculate the pH of a 1 x 10–8 M solution of HCl.
Q8. What do you know about pH-scale ? Most Important
Q9. Compare the acidic strengths of halogen acids HF, HCl, HBr and HI.
Q10. Explain Friedal Craft acylation in detail.
Q11. Explain Markovnikov Rule in Detail. Most Important
Q12. Explain Ozonolysis in Detail
HBSE Class 11 Chemistry 3 Marks Important Questions with Answer 2023
Q1. How much copper can be obtained from 100 gram of CuSO4 ?
Q2. Would you expect the Ionization enthalpies for two isotopes of the same element to be same or different? Justify your answer.
Q3. Complete the following chemical reactions :
(A) PbS(s) + H2O(aq) →
(B) CaO(s) + H2O(g) →
(C) AICl3(g) + H2O(l) →
Q4. Why Be shows anomalous behaviour?
Q5. What do you know about allotropes of Carbon ?
Q6. Define the following terms:
(i) Modern periodic law
(ii) Shielding effect
(iii) Atomic radius
Q7. Define the terms :
(i) Molar enthalpy of fusion (ΔHo fus.)
(ii) Hess’s Law
(iii) Entropy
Q8. Define the following:
(i) Mole fraction
(ii) Molarity
(iii) Molality
Q9. What do you understand by the following? Most Important
(a) inert pair effect
(b) allotropy
(c) catenation
Q10. What is enthalpy of solution? Explain in detail.
Q11. How do electronegativity vary in a group and period ? Explain.
Q12. What do you know about Inductive Effect?
Q13. Describe the hybridization in SF6 molecule.
Q14. The enthalpy of formation of CH4, CO2 and H2O are -74.8 kJ/mol, -393.5 kJ/mol and -285.8 kJ/mol respectively. Find Enthalpy of combustion of CH4.
Q15. Balance the given equation by ion electron method in acidic medium:
Fe2+(aq) + MnO4– (aq) → Fe3+(aq) + Mn2+(aq)
Q16. Balance the given equation in acidic medium using Half reaction method:
Fe2+ (aq) + Cr2O72– (aq) → Fe3+ (aq) + Cr3+ (aq)
Q17. Describe magnetic orbital quantum number.
Q18. Explain Pauli Exclusion principle with example. Most Important
Q19. Discuss the principle of estimation of sulphur present in an organic compound.
Q20. 0.2422 g of organic compound gives 0.3269 g of Barium sulphate. Find percentage of sulphur in the compound. ( Atomic weight Ba = 137, S = 32)
Q21. Convert the following:
(i) CH2=CH2 →OH—CH2—CH2—OH
(ii) CH3CH2OH → C2H4
(iii) CH3CH2Br → C2H4
Q22. A gas has a volume of 300 ml at 27°C and 730mm (Hg) pressure. What will be its volume at standard conditions of temperature and pressure ?
Q23. What do you mean by 10 volume H2O2? Give two uses of H2O2.
Q24. Discuss the Carius method for the estimation of sulphur in organic compound.
Q25. What is meant by Acidic Rain ? What are its consequences ? Most Important
Q26. What are limitations of Bohr’s model? Explain.
Q27. Explain mechanism of reaction of Cl2 (Chlorine) with CH4 (Methane).
Q28. Draw the cis and trans structures of hex-2-ene. Which isomer will have higher boiling point and why?
Q29. What do you know about Steam Distillation?
Q30. Justify giving reactions that among halogens, fluorine is the best oxidant and among hydrohalic compounds, hydroiodic (HI) acid is the best reductant.
Q31. 2.9 gram of a gas at 95°C occupied the same volume as 0.184 gram of H2 at 17°C at the same pressure. What is the molar mass of the gas?
Q32. Define open system, extensive properties and enthalpy.
Q33. Define acid and base with example in Arrhenius concept.
Q34. What is anti-Markovnikov’s rule ? Explain with example. Most Important
Q35. What is Markovnikov’s rule? Explain with example. Most Important
Q36. Explain Hund’s rule of maximum multiplicity with example.
Q37. Define closed system, intensive properties and internal energy.
Q38. Define acid and base with example in Bronsted-Lowry concept.
Q39. Explain Aufbau Principle.
Q40. Give IUPAC names of the following:
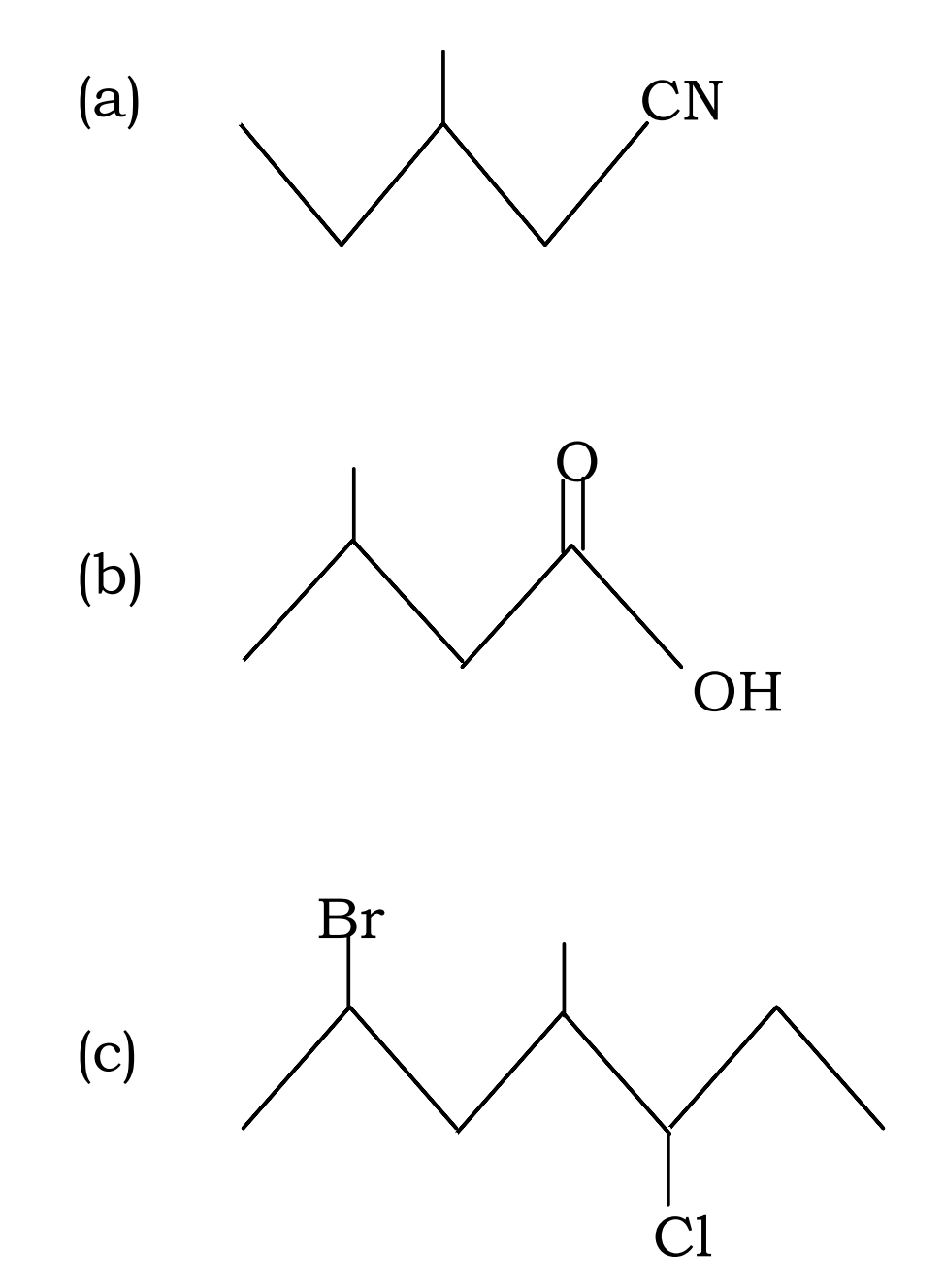
Q41. Give IUPAC names of the following :
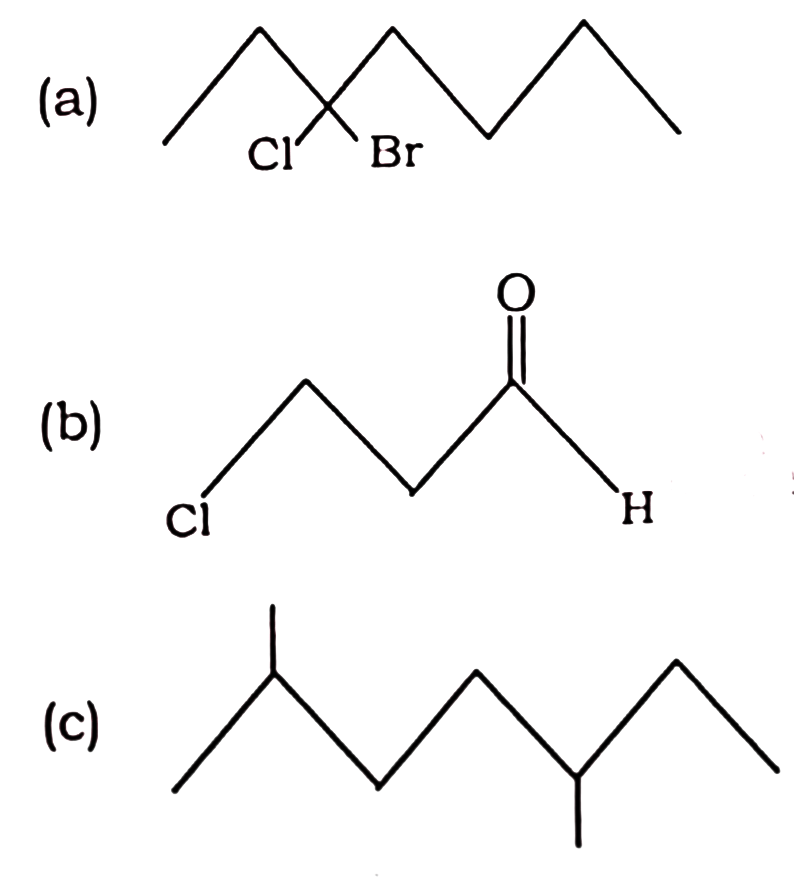
HBSE Class 11 Chemistry 5 Marks Important Questions with Answer 2023
Q1. Define and explain heat of reaction. What is Hess’s law of constant heat summation, explain with example?
Q2. Explain bond order and magnetic behaviour of oxygen molecule on the basis of molecular orbital theory.
Q3. What is meant by the term Bond Order? Calculate the bond order of N2, O2, O2+ and O2–. Most Important
Q4. What is Hybridisation ? On the basis of hybridization explain shapes of C2H2 and NH3 molecules.
Q5. What is meant by hybridization of atomic orbitals? Describe the shapes of sp, sp2 and sp3 hybrid orbitals with example. Most Important
Q6. Complete the following reactions :
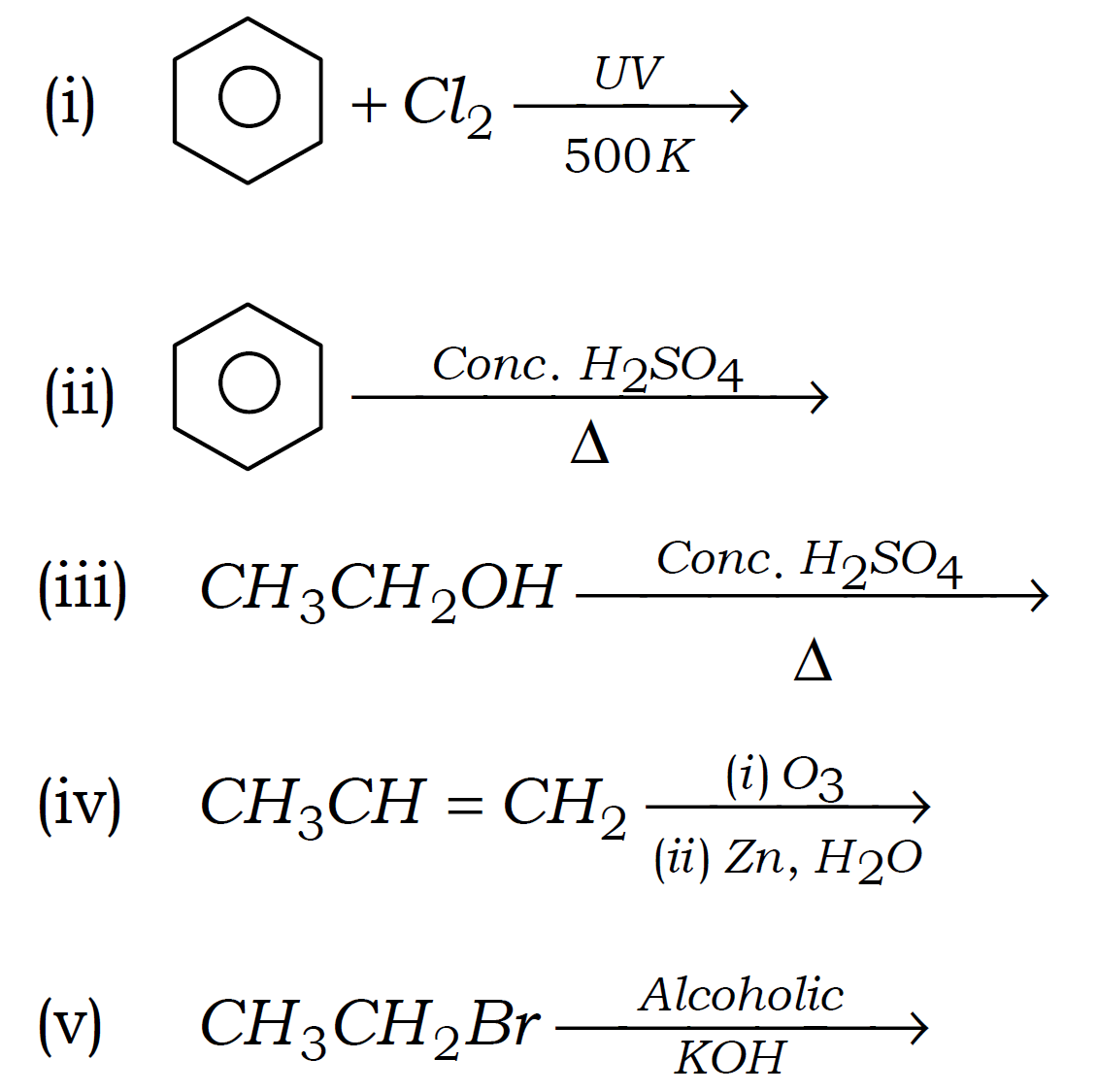
Q7. What do you known about Cathode Rays?
Q8. Write a detail note on Photoelectric Effect.
Q9. Explain in detail ‘bond enthalpy’ with example.
Q10. What is dipole moment? Explain in detail with an advantage.
Q11. Define pH scale and explain in detail.
Q12. What do you know about Arrhenius concept of acids and bases? Explain in detail with example.