Class 11 Chemistry BSEH Solution for MCQ Important Question Answer for Haryana board. CCL Chapter Provide Class 1th to 12th all Subjects Solution With Notes, Question Answer, Summary and Important Questions. Class 11 mcq, summary, Important Question Answer, Textual Question Answer in hindi medium and english medium are available of HBSE Board.
- Also Read – HBSE Class 11 All Subject Important Questions
HBSE Class 11 Chemistry Important MCQ Question with Answer / objective Question Answer for Haryana Board Solution.
HBSE Class 11 Chemistry Important MCQ Question Answer 2023
1. Which of the following will have largest number of atoms?
(A) 56 gram Fe(s)
(B) 2.7 gram Al(s)
(C) 0.16 gram O2(g)
(D) 1 gram He(g)
Answer
Ans – (D) 1 gram He(g)
2. The number of orbitals in a sub-shell is equal to :
(A) 2l
(B) 2l + 1
(C) 2l + 2
(D) l + 2
Answer
Ans – (B) 2l + 1
3. Which is the correct value of concentration of copper in drinking water?
(A) 0.3 PPm
(B) 0.2 PPm
(C) 3.0 PPm
(D) 2 PPm
Answer
Ans – (C) 3.0 PPm
4. Which of the following is not Lewis acid?
(A) Ag+
(B) AICI3
(C) BF3
(D) Al2Cl6
Answer
Ans – (D) Al2Cl6
5. What is number of oxidation Mn-atom in KMnO4 ?
(A) +5
(B) +7
(C) +3
(D) +1
Answer
Ans – (B) +7
6. The type of hybridization of carbon in CH4 is:
(A) sp
(B) sp2
(C) sp3
(D) dsp2
Answer
Ans – (C) sp3
7. What is the hybridization of boron in Diborane ?
(A) sp3
(B) sp2
(C) sp
(D) sp3d
Answer
Ans – (A) sp3
8. In H₂O molecule, the correct hybrid state of Oxygen is :
(A) sp
(B) sp2
(C) sp3
(D) dsp2
Answer
Ans – (C) sp3
9. In CO2 (Carbon Dioxide) molecule the carbon is in which hybrid state?
(A) sp
(B) sp2
(C) sp3
(D) dsp2
Answer
Ans – (A) sp
10. Which carbonate is thermally most stable ?
(A) MgCO3
(B) CaCO3
(C) BaCO3
(D) SrCO3
Answer
Ans – (C) BaCO3
11. Thermodynamically the most stable form of Carbon is : Most Important
(A) Diamond
(B) Graphite
(C) Fullerenes
(D) Coal
Answer
Ans – (B) Graphite
12. Mg reacts with dil. HNO3 giving:
(A) NO
(B) NO₂
(C) N₂O
(D) NH4+
Answer
Ans – (C) N₂O
13. The treatment of Pb with dil. HNO3 produces?
(A) NH4+
(B) N₂O
(C) NO
(D) NO₂
Answer
Ans – (C) NO
14. Which of the following is not isoelectronic species ?
(A) Na+
(B) O–
(C) F–
(D) Mg++
Answer
Ans – (B) O–
15. Which species is smallest in size?
(A) F
(B) O2-
(C) Al3+
(D) Mg2+
Answer
Ans – (C) Al3+
16. The number of Pi (π) bonds present in C2H2 is:
(A) 2
(B) 1
(C) Zero
(D) 3
Answer
Ans – (A) 2
17. Number of significant figures in 2.005 are:
(A) 1
(B) 2
(C) 3
(D) 4
Answer
Ans – (D) 4
18. Which one has zero value of dipole moment?
(A) H2O
(B) NH3
(C) CCl4
(D) CH4
Answer
Ans – (D) CH4
19. In the following superoxide is :
(A) Na2O2
(B) Li2O
(C) KO2
(D) None of these
Answer
Ans – (C) KO2
20. Conjugate acid for Bronsted base HCO3– is:
(A) H2CO3
(B) CO32-
(C) OH–
(D) CO2
Answer
Ans – (A) H2CO3
21. Oxidation number of chromium in Cr2O72- ion is:
(A) 4
(B) +3
(C) -2
(D) +6
Answer
Ans – (D) +6
22. In the following water gas is :
(A) CO + N2
(B) CO + H2
(C) H2 + Cl2
(D) CH4
Answer
Ans – (B) CO + H2
23. Electrophile is:
(A) C2H5O–
(B) OH–
(C) NC–
(D) C+H3
Answer
Ans – (D) C+H3
24. 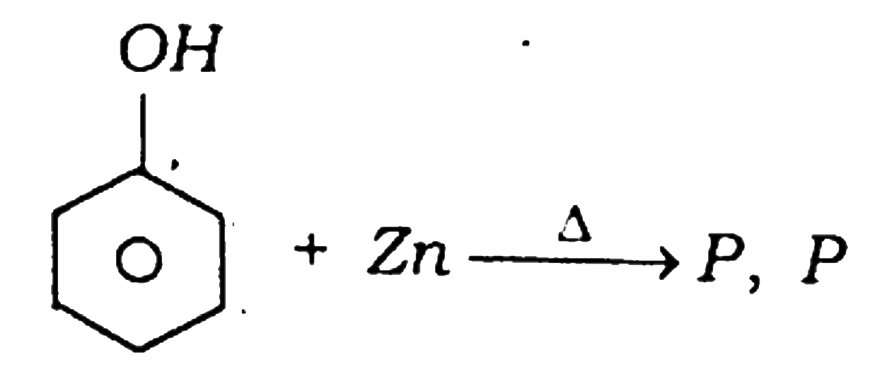 is :
is :
(A) Benzene
(B) Toluene
(C) Cyclohexane
(D) None of these
Answer
Ans – (A) Benzene
25. Isoelectronic ions are:
(A) Na+, O–
(B) F, O2–
(C) Al3+, P3+
(D) F–, O2–
Answer
Ans – (D) F–, O2–
26. Electronic configuration of d-block elements is :
(A) (n–1)d1–10ns2
(B) (n–1)d1-10ns0-2
(C) (n–1)d1–10ns1–2
(D) None of these
Answer
Ans – (C) (n–1)d1–10ns1–2
27. Number of Neutrons in 8035Br are:
(A) 80
(B) 35
(C) 45
(D) 115
Answer
Ans – (C) 45
28. Oxidation state of Mn in MnO42– is:
(A) +7
(B) +6
(C) -2
(D) +2
Answer
Ans – (A) +7
29. In which dipole moment is zero ?
(A) SnCl2
(B) SO2
(C) H2O
(D) CCl4
Answer
Ans – (D) CCl4
30. Which compound shows zero dipole moment?
(A) CCl4
(B) H2O
(C) SO2
(D) H2S
Answer
Ans – (A) CCl4
31. Which compound do not show zero dipole moment?
(A) CO2
(B) BF3
(C) H2O
(D) CCl4
Answer
Ans – (B) BF3
32. In equation 2 Cl (g) → Cl2 (g) the value of ΔH and ΔS will be :
(A) +ve, –ve
(B) +ve, +ve
(C) –ve, –ve
(D) –ve, +ve
Answer
Ans – (C) –ve, –ve
33. At constant pressure:
(Α) ΔΗ = qν
(B) ΔH = 0
(C) ΔH = ΔU – PΔV
(D) ΔH = qp
Answer
Ans – (D) ΔH = qp
34. For the process to occur under adiabetic conditions, the correct condition is:
(A) ΔT = 0
(B) ΔP = 0
(C) q = 0
(D) w = 0
Answer
Ans – (C) q = 0
35. Which is hard ( heavy ) water ? Most Important
(A) H2O2
(B) H2O
(C) D2O
(D) D2O2
Answer
Ans – (C) D2O
36. Standard electrode potential of hydrogen is taken as: Most Important
(A) 0.0V
(B) –3.04V
(C) +2.85V
(D) 1.0 V
Answer
Ans – (A) 0.0V
37. Value of BOD in clean water will be:
(A) More than 5 ppm
(B) Less than 5 ppm
(C) More than 17 ppm
(D) None of these
Answer
Ans – (B) Less than 5 ppm
38. Plaster of Paris is:
(A) CaSO4
(B) CaSO4. ½H20
(C) CaSO4.2H2O
(D) CaOCl2
Answer
Ans – (B) CaSO4. ½H20
39. What is formula of Baking Soda ?
(A) NaHCO3
(B) Na2CO3
(C) NaOH
(D) KOH
Answer
Ans – (A) NaHCO3
40. What is formula of Caustic Soda ? Most Important
(A) Ca(OH)2
(B) NaOH
(C) KOH
(D) Na2CO3
Answer
Ans – (B) NaOH
41. In the following Nucleophiles are :
(A) OH–, H2O
(B) CH3, NC–
(C) >C=O, C+H3
(D) OH–, R3C+
Answer
Ans – (A) OH–, H2O
42. Out of F, Cl, O and N elements, the correct of chemical reactivity in terms of oxidizing property is
(A) F>C>O>N
(B) F> O> CI>N
(C) Cl> F>O>N
(D) O> F> N > CI
Answer
Ans – (B) F> O> CI>N
43. Standard boiling point of water is
(A) 99.0°C
(B) 100.0°C
(C) 100.6°C
(D) 99.6°C
Answer
Ans – (B) 100.0°C
44. The pH of milk is : Most Important
(A) 7.4
(B) 6.8
(C) 6.4
(D) 7.8
Answer
Ans – (B) 6.8
45. The pH of Human Saliva is :
(A) 7.4
(B) 9.2
(C) 7.8
(D) 6.4
Answer
Ans – (A) 7.4
46. The oxidation number of Copper in Cu2O is:
(A) –1
(B) –2
(C) +1
(D) +2
Answer
Ans – (C) +1
47. The oxidation number of Mn in KMnO4 is:
(A) +1
(B) +5
(C) +7
(D) +3
Answer
Ans – (C) +7
48. The oxidation number of Nitrogen in NO3– is:
(A) –3
(B) +1
(C) +3
(D) +5
Answer
Ans – (D) +5
49. The oxidation number of Cl in KClO3 is:
(A) +5
(B) +3
(C) +1
(D) –1
Answer
Ans – (A) +5
50. The oxidation number of Sulphur in SO42– is:
(A) –2
(B) +6
(C) +3
(D) +4
Answer
Ans – (B) +6
51. How many isotopes Hydrogen has ? Most Important
(A) 4
(B) 3
(C) 2
(D) 5
Answer
Ans – (B) 3
52. Out of these ions which one does not perform any biological functions in our body?
(A) K+
(B) Na+
(C) Ca++
(D) Ra++
Answer
Ans – (D) Ra++
53. The electronegativity of F atom is:
(A) 3.5
(B) 4.5
(C) 3.0
(D) 4.0
Answer
Ans – (D) 4.0
54. The critical temperature (TC) of CO2 (Carbon Dioxide) gas is :
(A) 33.98°C
(B) 30.98°C
(C) 27.98°C
(D) 37.98°C
Answer
Ans – (B) 30.98°C
55. The temperature at which the volume of gas is zero ?
(A) 0°C
(B) 0 K
(C) 0°F
(D) None of these
Answer
Ans – (B) 0 K
56. Number of moles in 64 g of Oxygen are:
(A) 4
(B) 2.0
(C) 2.5
(D) 3.0
Answer
Ans – (A) 4
57. Number of moles in 22 gram of carbondioxide are:
(A) 1.0
(B) 1.5
(C) 2
(D) 0.5
Answer
Ans – (D) 0.5
58. Number of moles in 54g of Water are :
(A) 2.0
(B) 2.5
(C) 3.0
(D) 3.5
Answer
Ans – (C) 3.0
59. Find number of unpaired electrons in Nitrogen atom:
(A) 1
(B) 2
(C) 3
(D) 4
Answer
Ans – (C) 3
60. Find number of unpaired electrons in Oxygen atom:
(A) 1
(B) 2
(C) 3
(D) 4
Answer
Ans – (B) 2
61. Arrange B, C, N, F and Si in correct order of their non-metallic character.
(A) B > C > Si > N > F
(B) Si > C > B > N > F
(C) F > N > C > B > Si
(D) F > N > C > Si > B
Answer
Ans – (C) F > N > C > B > Si
62. How many horizontal rows in Modern periodic table?
(A) 2
(B) 6
(C) 7
(D) 8
Answer
Ans – (C) 7
63. How many vertical columns in Modern Periodic table ?
(A) 2
(B) 8
(C) 32
(D) 18
Answer
Ans – (D) 18
64. Which type of intermolecular force exists in H2S molecules ?
(A) Dipole-dipole forces
(B) Dipole-induced dipole forces
(C) Dispersion forces
(D) Hydrogen bond
Answer
Ans – (A) Dipole-dipole forces
65. Which type of intermolecular force exists in Cl2 and CCl4?
(A) dipole-dipole forces
(B) dipole-induced dipole forces
(C) dispersion forces
(D) hydrogen bonds
Answer
Ans – (C) dispersion forces
66. A real gas acts as an ideal gas under which condition ?
(A) High temperature, low pressure
(B) Low temperature, high pressure
(C) High temperature, high pressure
(D) Low temperature, low pressure
Answer
Ans – (A) High temperature, low pressure
67. The enthalpies of all elements in their standard states are:
(A) Unity
(B) Zero
(C) <0
(D) >0
Answer
Ans – (B) Zero
68. The hydration enthalpies of alkali metal ions show following order
(A) Li+ > Na+ > K+> Rb+
(B) Li+ < Na+ < K+ < Rb+
(C) K+ > L+ > Rb+ > Na+
(D) K+ < Li+ < Na+ < Rb+
Answer
Ans – (A) Li+ > Na+ > K+> Rb+
69. Arrange B, Al, Mg and Kin correct order of their metallic character:
(A) B > Al > Mg > K
(B) Al > Mg > B > K
(C) Mg > Al > K > B
(D) K > Mg > Al > B
Answer
Ans – (D) K > Mg > Al > B
70. Which metal is used to prepare H2 with dil. H2SO4 ?
(A) Cu
(B) Hg
(C) Ag
(D) Zn
Answer
Ans – (D) Zn
71. Which alkali metal gives hydrated salt?
(A) Li
(B) Na
(C) K
(D) Cs
Answer
Ans – (A) Li
72. CnH2n is general formula of :
(A) alkene
(B) alkane
(C) alkyne
(D) arene
Answer
Ans – (B) alkane
73. CnH2n-2 is general formula of:
(A) Alkanes
(B) Arenes
(C) Alkenes
(D) Alkynes
Answer
Ans – (D) Alkynes
74. Heating a mixture of sodium benzoate with soda-lime gives:
(A) Methane
(B) Benzene
(C) Ethane
(D) Propane
Answer
Ans – (B) Benzene
75. Heating a mixture of sodium acetate with soda-lime gives :
(A) Propane
(B) Ethane
(C) Methane
(D) Butane
Answer
Ans – (C) Methane
76. Which alkali metal having least melting point?
(A) Na
(B) K
(C) Rb
(D) Cs
Answer
Ans – (D) Cs
77. Which of the following organic compounds is not aromatic?
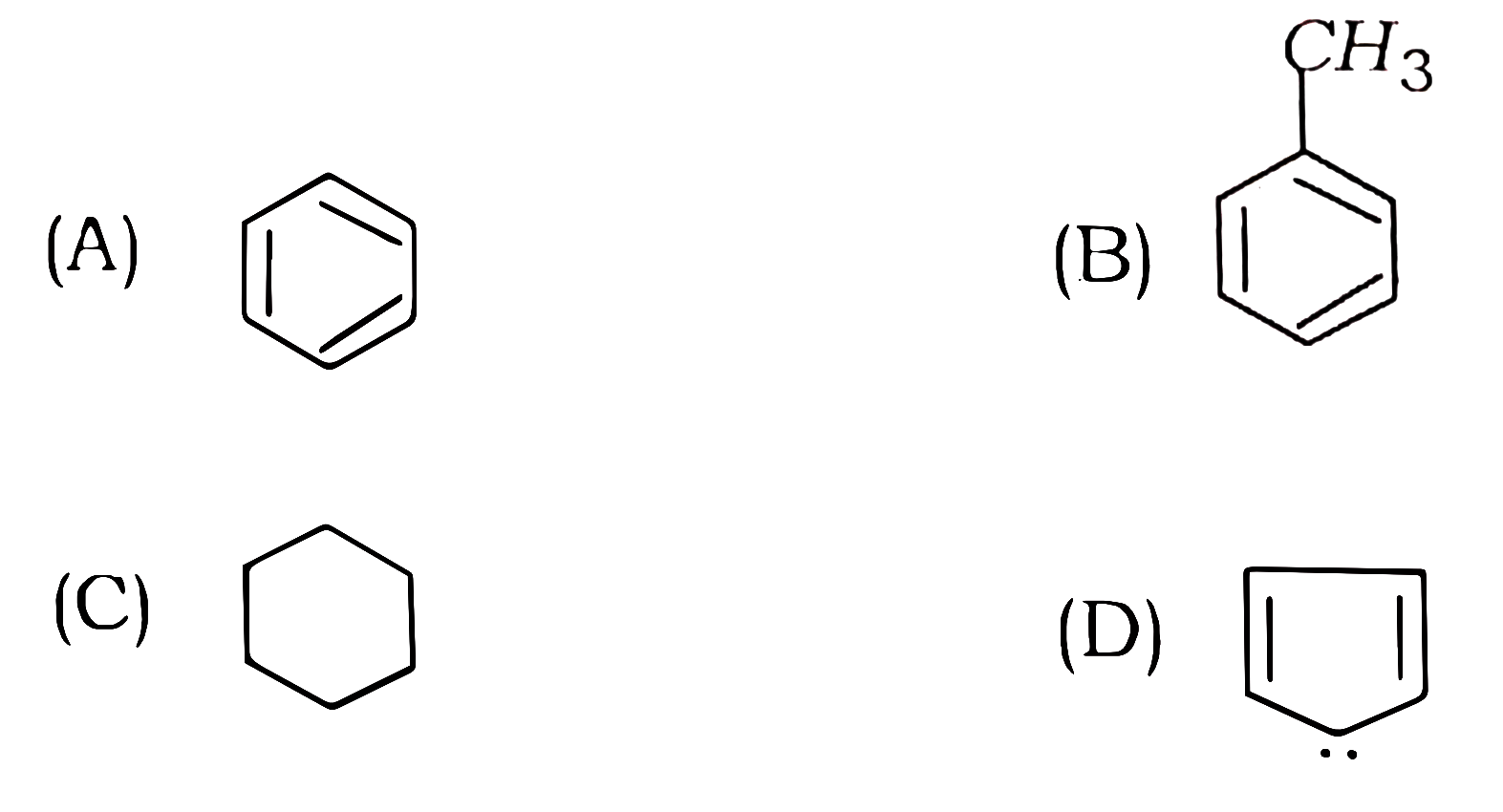
Answer
Ans – (C)
78. Which is aromatic out of these compounds?

Answer
Ans – (C)
79. Which formula is not correct?
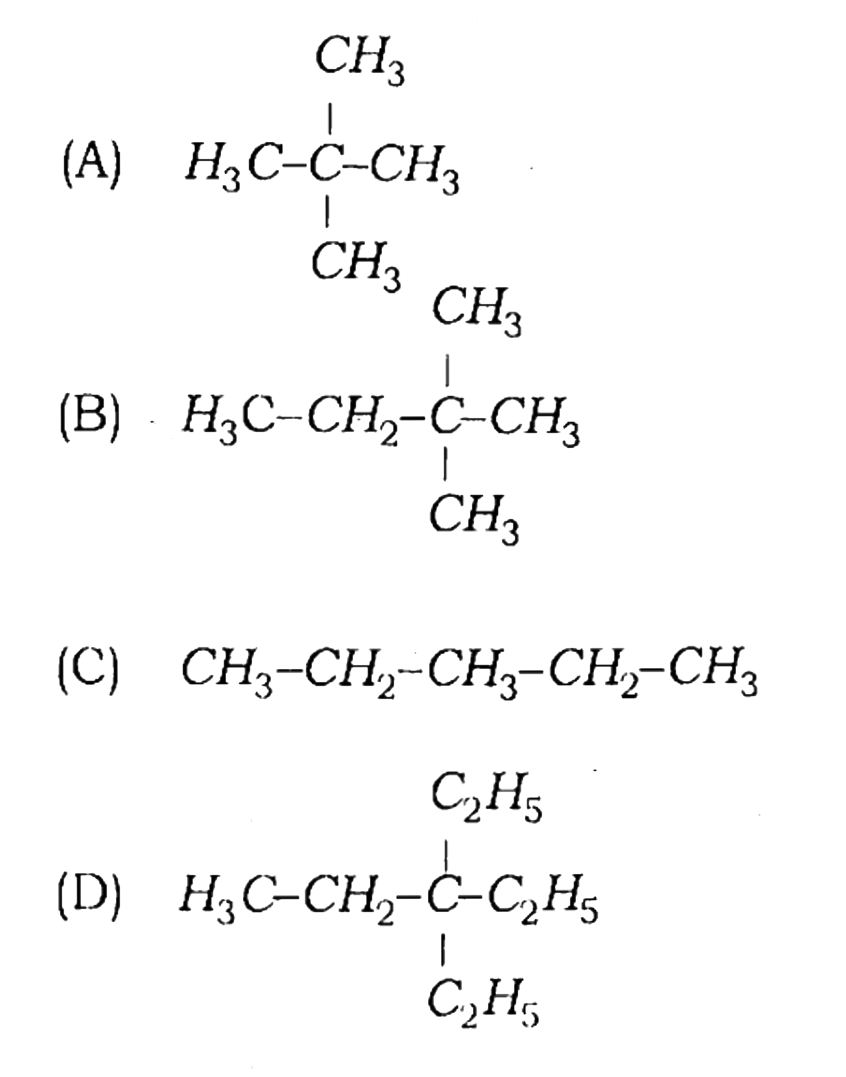
Answer
Ans – (C)
80. Which of the following carbanion is most stable?
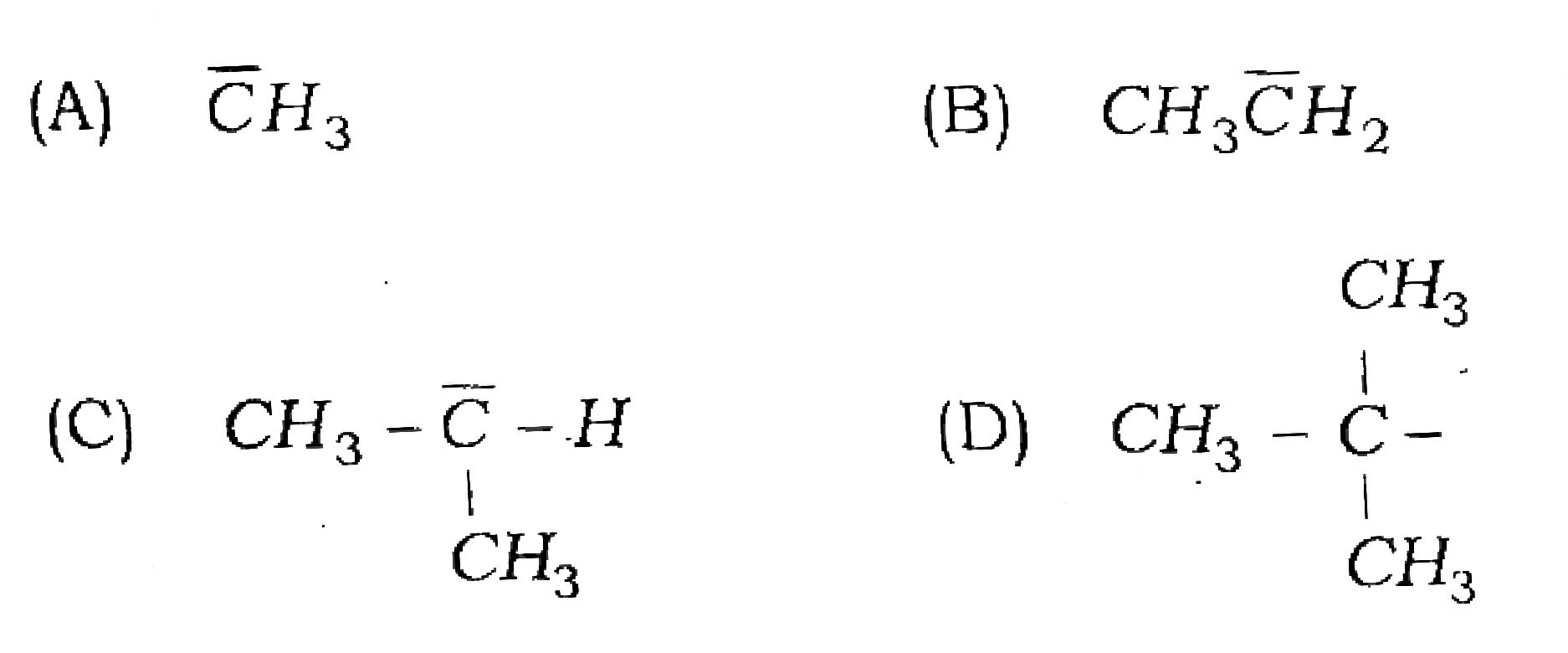
Answer
Ans – (A)