Class 12 Chemistry Important Question Answer solution with pdf. Here We Provides Class 1 to 12 all Subjects NCERT Solution with Notes, Question Answer, CBSE and HBSE Important Questions, MCQ and old Question Papers for Students.
- Also Read :- HBSE Class 12 Important Questions [Latest]
HBSE ( Haryana Board ) Solution of Class 12 Chemistry important Question And Answer solution for 2025 exams.
HBSE Class 12 Chemistry Important Questions 2025
HBSE Class 12 Chemistry Chapter 1 – Solutions Important Questions 2025
Q1. 1.00 g of a non-electrolyte solute dissolved in 50 g of benzene lowered the freezing point of benzene by 0.40 K. The freezing point depression constant of benzene is 5.12 K kg mol-1. Find the molar mass of the solute.
Q2. Define the following terms: (i) Molality (ii) Osmotic pressure
Q3. If the density of lake water is 1.25 g kg of water. Calculate the molality of m L-1 Na+ and contains 92 g of Na+ ions per ions in lake.
Q4. 4.0 gm of NaOH, dissolved in 500 ml of solution, calculate molarity of the solution.
Q5. What role does the Molecular interaction play in a solution of alcohol and water?
HBSE Class 12 Chemistry Chapter 2 – Electrochemistry Important Questions 2025
Q1. What is Kohlrausch law? Discuss its applications with examples.
Q2. A solution of CuSO4 is electrolysed for 10 minutes with a current of 1.5 amperes. What is mass of copper deposited at Cathode ? (Cu63.5)
Q3. If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire ?
Q4. What is Corrosion ? Give factors which promotes corrosion and name the methods to prevent corrosion.
OR
What is Corrosion ? Explain methods to prevent corrosion.
Q5. A solution of Ni(NO3)2 is electrolysed between platinum electrode using a current of 5 ampere for 20 min., what mass of Ni is deposited at cathode ? (Ni = 58.7) Most Most Important
Q6. How much charge required in Coulomb for the following reductions or oxidations ?
- 1 mole of Al3+ to Al
- 1 mole of H2O to O2
- 1 mole of MnO4– to Mn2+
- 1 mole of Cu2+ to Cu
- 1 mole FeO to Fe2O3
- 1 mole of MnO42- to MnO2
- 1 mole of Pb4+ to Pb2+
- 1 mole of CaCl2 to Ca
- 1 mole of MnO4– to MnO2
- 1 mole of Fe3+ to Fe2+
- 1 mole of ZnO to Zn
- 1 mole of CrO3 to Cr3+
Q7. What is electrochemical series ? Give its three applications.
HBSE Class 12 Chemistry Chapter 3 – Chemical Kinetics Important Questions 2025
Q1. Define half life period and rate of reaction.
Q2. Calculate the half life of a first order reaction from rate constant 200 sec-1.
Q3. What is Activation Energy?
Q4. A first order reaction has a rate constant 1.15 x 10-3 S-1 . How long will 5 g of this reactant take to reduce to 3 g?
Q5. Define collision frequency.
Ans – The number of collisions per second per unit volume of the reaction mixture is known as collision frequency (Z).
HBSE Class 12 Chemistry Chapter 4 – The d and F block Elements Important Questions 2025
Q1. Why do transition elements exhibit tendency for complex formation ? Explain with example.
Q2. What is Lanthanide contraction ? Explain its reasons and consequences.
Q3. The transition metals generally form coloured compounds. Give reasons.
Q4. Describe the oxidising action of K2Cr2O7 and the ionic equations for its reaction with: (a) iodide ion (b) iron (II) solution (c) H2S.
Q5. Transition Metals are form Interstitial compounds Explain.
Q6. Explain giving reasons transition metals and their many compounds act as good catalyst.
Q7. Explain giving reason:
(i) Transition metal and many of their compounds show paramagnetic behaviour.
(ii) The enthalpy of atomization of the transition metal are high.
(iii) The transition metal generally forms coloured compounds.
(iv) Transition metal acts as good catalyst..
(v) Draw the structure of MnO4– Ion.
HBSE Class 12 Chemistry Chapter 5 – Coordination Compounds Important Questions 2025
Q1. What are ambidentate ligands ?
Q2. Explain Co-ordination isomerism with example.
Q3. Write IUPAC names of following:
(1) [Co(NH3)6]Cl3
(2) [Co(NH3)5Br]SO4
(3) [Pt(H2O)2BrNO2]
(4) [Ni(CO)4]
(5) K3[Fe(CN)6]
(6) K3[Fe(C2O4)3]
(7) K3[Al(C2O4)3]
(8) K3[Cr(C2O4)3]
(9) [Cr(NH3)3(H2O)3]Cl3
(10) Zn2[Fe(CN)6]
(11) [Pt(NH3)4NO2Cl]SO4
(12) [Co(NH3)4Cl(NO2)]CI
(13) K4[Mn(CN)6]
(14) (p)O2N-C6H4-OCH3
Ans –
(1) [Co(NH3)6]Cl3 → Hexaamminecobalt(III) chloride
(2) [Co(NH3)5Br]SO4 → Pentammine bromo cobalt (III) sulphate
(3) [Pt(H2O)2BrNO2] → Diammineaquabromonitrito-O-platinum(II)
(4) [Ni(CO)4] → Tetracarbonylnickel(0)
(5) K3[Fe(CN)6] → potassium hexacyanoferrate(III)
(6) K3[Fe(C2O4)3] → Potassium trioxalatoferrate(III)
(7) K3[Al(C2O4)3] → Potassium trioxalatoaluminate(III)
(8) K3[Cr(C2O4)3] → Potassium trioxalatochromate(III)
(9) [Cr(NH3)3(H2O)3]Cl3 → Tris(ammine)triaquachromium(III) chloride
(10) Zn2[Fe(CN)6] → zinc hexacyanoferrate(II)
(11) [Pt(NH3)4NO2Cl]SO4 → Tetraamminechloronitritoplatinum(II) sulfate
(12) [Co(NH3)4Cl(NO2)]CI → Tetraamminechloridonitrocobalt(III) chloride
(13) K4[Mn(CN)6] → potassium hexacyanomanganate(II)
(14) (p)O2N-C6H4-OCH3 → 4-methoxy-1-nitrobenzene
HBSE Class 12 Chemistry Chapter 6 – Haloalkanes and Haloarenes Important Questions 2025
Q1. What are ambident nucleophiles ? Explain with an example.
Q2. Explain with example Friedel-Crafts alkylation of Methoxybenzene.
Q3. What is Wurtz reaction?
Ans –Alkyl halides react with sodium in dry ether to give hydrocarbons containing double the number of carbon atoms present in the halide. This reaction is known as Wurtz reaction.
2RX + 2Na → RR + 2NaX
Q4. Convert Propanone to Propene.
HBSE Class 12 Chemistry Chapter 7 – Alcohols, Phenols and Ethers Important Questions 2025
Q1. Write the IUPAC names of following compounds:
(1) CH3–CHOH–CH2–CHOH–CH3 →
(2) C6H5–O–C6H5 →
(3) C6H5CH2CH2Cl →
(4) (CH3CH2)2NCH3 →
(5)  →
→
(6)  →
→
(7)  →
→
Q2. Explain with example Kolbe’s reaction.
Q3. Explain with example Reimer-Tiemann reaction.
Q4. Write the mechanism of acid dehydration of ethanol to yield ethene.
Q5. CH3CH2OH
HBSE Class 12 Chemistry Chapter 8 – Aldehydes, Ketones and Carboxylic Acids Important Questions 2025
Q1. Describe Aldol condensation reaction.
Q2. Describe Cross Aldol Condensation reaction.
Q3. Describe Decarboxylation reaction.
Q4. Describe the following: (i) Hell-Volhard-Zelinsky Reaction (ii) Cannizzaro reaction (iii) Rosenmund reduction (iv) Stephen reaction (v) Clemmensen reduction (vi) Wolff-Kishner reduction (vii) Esterification
Q5. What is Cannizaro’s reaction ? Write its mechanism ?
Ans –
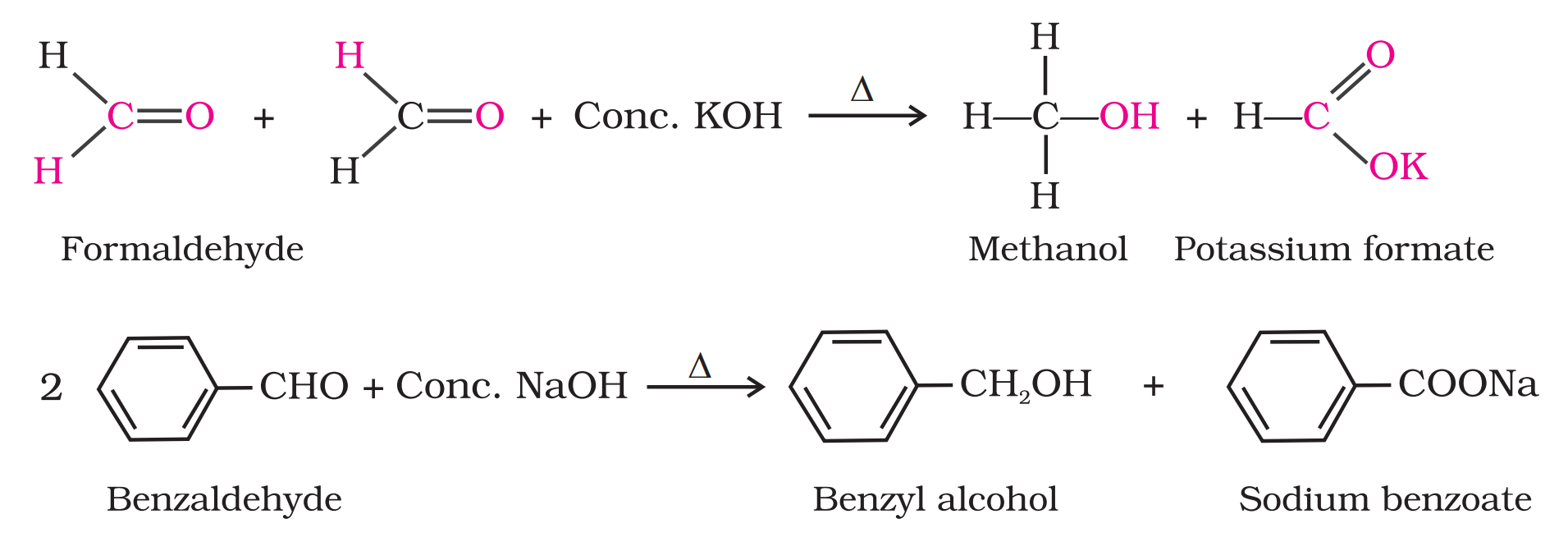
Q6. Convert Benzene into :
(i) Acetophenone
(ii) Benzaldehyde
(iii) Benzophenone
Q7. Write the products of the following reactions:
(i) 
(ii) 
HBSE Class 12 Chemistry Chapter 9 – Amines Important Questions 2025
Q1. Write a short note on carbylamine reaction.
Q2. What is Gabriel Phthalimide synthesis reaction?
Q3. Write short note on Hofmann’s bromamide degradation reaction.
Q4. Write a short note on Hofmann’s bromamide reaction.
Q5. Write a short note on coupling reaction.
HBSE Class 12 Chemistry Chapter 10 – Biomolecules Important Questions 2025
Q1. Write the names of two vitamins soluble in fats.
Q2. Define the terms :
(i) Biomolecules
(ii) Carbohydrates
(iii) Reducing Sugars
Q3. What are essential and non-essential amino acids ? Give one example of each type.
Q4. Define the following terms:
(a) Anomer
(b) Peptide Bond OR Peptide linkage (c) Reducing sugar
Q5. Write four differences between DNA and RNA.