NCERT Solution of Class 10 Science Important MCQ Question Answer solution with pdf. Here We Provides Class 1 to 12 all Subjects NCERT Solution with Notes, Question Answer, CBSE and HBSE Important Questions, MCQ and old Question Papers for Students.
- Also Read :- Class 10 Science NCERT Solution
HBSE ( Haryana Board ) Solution of Class 10th Science all chapters important Question And Answer of Physics, Chemistry and Biology Important Question Solution.
Class 10 Science Important Question Answer for 2024 Chapter-Wise
Table of Contents
HBSE Class 10 Science Chemistry Portion Important Questions 2024
HBSE Class 10 Science Chapter 1 – Chemical Reaction and Equations Important Questions 2024
Q1. What do you understand by endothermic reactions? Give one example.
Q2. What do you mean by combination reactions? Give one example. Most Important
Q3. What do you mean by displacement reaction? Give one example. Most Important
Q4. What do you mean by decomposition reactions? Give one example (chemical equation).
Q5. What do you mean by Precipitation reaction? Give one example.
Q6. Write the balanced chemical equation for the following reaction:
Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
Q7. Write one equation each for decomposition reactions where energy is supplied in the form of heat and light.
Q8. Why does the colour of copper sulphate solution change when an iron nail is dipped in it? Give chemical equation for it.
Q9. Write the balanced chemical equation for the following reaction:
Aluminium + Copper Chloride → Aluminium Chloride + Copper
Q10. Fat and oil containing foods are packed with which gas and why?
Q11. Why should Mg ribbon be cleaned before burning in air ?
Q12. What do you mean by double displacement reactions? Give one example (chemical equation).
Q13. What is rancidity?
Q14. Why is respiration considered as exothermic reaction? Explain.
Q15. What is a balanced chemical equation? Why should chemical equations be balanced?
Q16. Which reactant is Oxidised in the following chemical reaction?
CuO + H2 → Cu + H2O
Q17. Which of the following reactant is reduced in chemical reaction?
Cuo + H2 → Cu + H2O
Q18. What is the colour of Ferrous sulphate crystals? How does this colour change after heating ?
Q19. What is redox reaction ? Give an example.
HBSE Class 10 Science Chapter 2 – Acid, Bases and Salt Important Questions 2024
Q1. What happens when gypsum is heated to 373 K ? Give chemical equation for it.
Q2. What is the common name of CaOCl2 ? Give the chemical reaction by which it is prepared.
Q3. What is Acid Rain? How does it affect aquatic life?
Q4. What is chlor-alkali process? Explain with the help of a chemical equation. Most Important
Q5. Why does distilled water not conduct electricity, whereas rain water does ?
Q6. While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid ?
Q7. What is neutralisation reaction ? Give one example. Most Important
Q8. Why should curd and sour substances not be kept in Brass and Copper vessels ?
Q9. Write common name and two uses of NaHCO3.
Q10. Which Acid is present in tamarinds?
Q11. What is chemical formula of baking soda? Give chemical equation used in its preparation. Most Important
Q12. What is the chemical formula of washing soda ? Give chemical equation used in its preparation.
Q13. Give two important uses of washing soda and baking soda.
Q14. Why do acids not show acidic behaviour in the absence of water?
Q15. What is pH scale in chemistry? Explain.
Q16. Four solution A, B, C and D when tested with a universal indicator, showed pH as 1, 7, 6 and 13 respectively Which solution is neutral, strongly alkaline, weak alkaline or weak acid and strongly acidic ?
HBSE Class 10 Science Chapter 3 – Metals and Non-Metals Important Questions 2024
Q1. Explain reactivity series with examples. Most Important
Q2. Explain the extraction of metals low in reactivity series.
Q3. Write constituents of Bronze and Brass.
Q4. What are amphoteric oxides ? Give two examples of amphoteric oxides. Most Important
Q5. Write brief note on Electrolytic Refining. Most Important
Q6. In which state ionic compound exists? Why do they have high melting point ? Most Important
Q7. Write electronic configuration of Aluminium (At. No. – 13 ) and Sulphur (At. No. – 16).
Q8. Why is sodium kept in kerosene oil?
Q9. Differentiate between Alloy and Amalgam.
Q10. What is thermit reaction? Give chemical equation for it. Most Important
Q11. Identify the metals and metalloids in the following elements:
Sodium ( Na), Silicon ( Si), Germanium (Ge), Lithium (Li).
Q12. Define the following:
(a) Mineral
(b) Gangue
Q13. Write the chemical equation for the following reactions:
(a) Iron with steam
(b) Calcium with water
Q14. Explain the following in context of metals :
(i) Malleability
(ii) Ductility
(iii) Conductor of heat and electricity
(iv) Sonorous
Q15. Differentiate between Calcination and Roasting.
Q16. Name two metals which will displace hydrogen from dilute acids and two metals which will not.
Q17. Name a non-metal that possesses lustuer.
Q18. Name a metal which is liquid at room temperature.
Q19. What happens when metals are burnt in air ? Give one example.
Q20. What happens when metals react with acids ? Give one example.
Q21. What happens when metals react with water ? Give one example.
Q22. What happens when water soluble metal oxides are dissolved in water? Give one example.
Q23. State two ways to prevent the rusting of iron.
Q24. Differentiate between metal and non-metal on the basis of their chemical properties.
Q25. Explain the meanings of malleable and ductility with example.
Q26. Write a short note on electrolytic refining of Copper.
HBSE Class 10 Science Chapter 4 – Carbon and its Compound Important Questions 2024
Q1. Explain the following processes with help of chemical reactions: Most Important
(a) Esterification
(b) Saponification
(c) Hydrogenation
Q2. Explain in detail the Nomenclature of carbon compounds. Most Important
Q3. What happens when ethanol reacts with the following: Most Important
(a) Acidified potassium dichromate
(b) Sodium
(c) Hot. Conc. H2SO4
Q4. Detergents are effective in Hard Water also. Comment.
Q5. Explain the mechanism of cleaning action of soaps. Most Important
Q6. Give two differences between soaps and detergents.
Q7. Draw the structures of all possible isomers of pentane. Most Important
OR
Draw all the structural isomers of pentane (C5H12).
Q8. Draw the structures for the following compounds:
(a) Ethanoic acid
(b) Hexanal
(c) Butanone
(d) Butane
Q9. What is the difference between two consecutive homologous ?
(a) In terms of molecular mass
(b) Number of carbon and hydrogen atoms
(c) Chemical properties
Q10. How would you distinguish experimentally between an alcohol and carboxylic acid ?
Q11. Write two uses of Ethanol.
Q12. Explain with examples the following properties of carbon compounds: Most Important
(a) Oxidation Reaction
(b) Addition Reaction
(c) Substitution Reaction
Q13. What do you mean by Oxidising agent? Why is conversion of ethanol to ethanoic acid an oxidation reaction? Give chemical reaction also.
Q14. Alcohol is a clean fuel. Comment.
Q15. Name the functional group present in Butanone.
Q16. Give chemical equation for the reaction of ethanoic acid with following: Most Important
(a) NaOH
(b) Na2CO3
(c) NaHCO3
(d) CH3CH2OH in the presence of acid
Q17. Write the names of the following compounds : Most Important
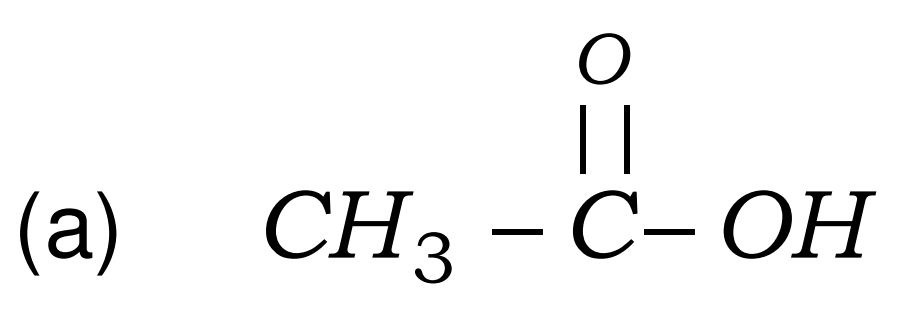
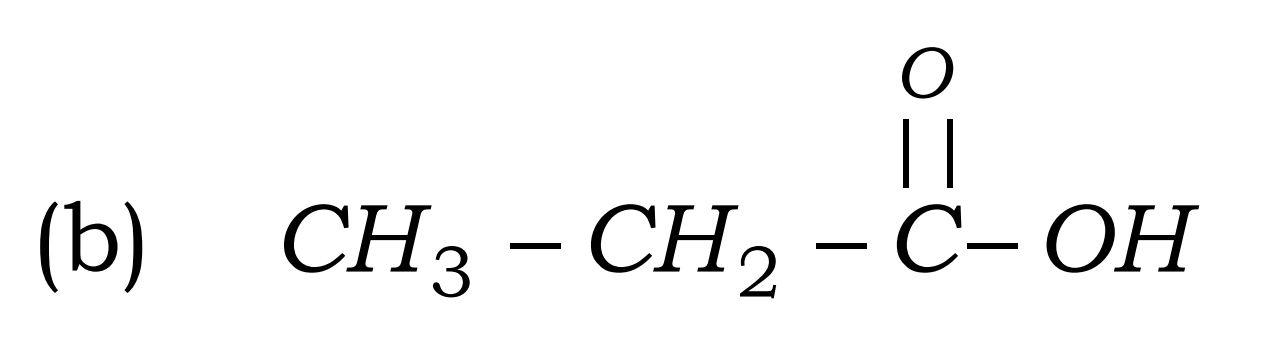
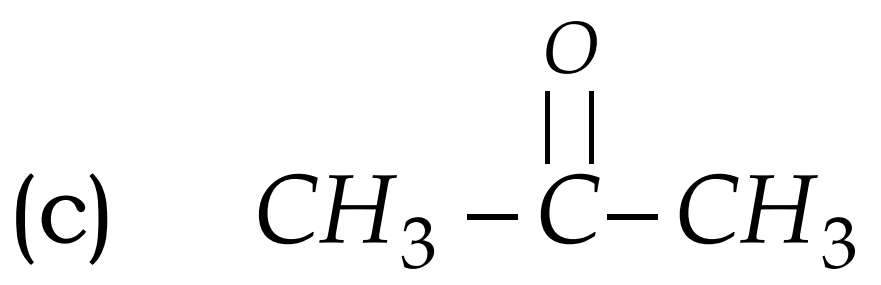
- CH3-CL
- CH3-CH2-CH2-OH
- CH3 – CH2 – Cl
- CH3 – CH2 – OH
- CH3 – CH2 -CHO
- CH3-CH2-CH2– Br
- CH3-C≡CH
- CH3-OH
- CH3-CH=CH2
- CH3-CH2-Br
- CH3COOH
- CH3Br
- CH3COCH3
- CH3CHO
- CH3-CH2-COOH
- CH3-CH2-CH2-CH3
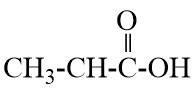
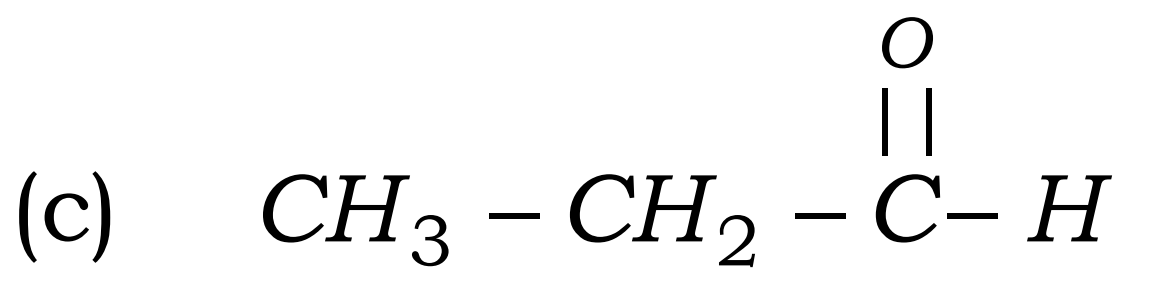
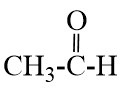
Q18. What is hydrogenation? What is its industrial application?
Q19. What is saponification reaction? Give example.
Q20. Which of the following hydrocarbons undergo addition reaction?
C2H6, C3H8, C3H6, C2H2
Q21. Define homologous series and functional group. Most Important
Q22. What are saturated and unsaturated hydro-carbons? Most Important
Q23. Explain two properties of carbon atom which lead to the huge number of carbon compounds.
Q24. Write the formula of benzene and draw its structure. Most Important
Q25. Differentiate between Hard water and Soft water.
Q26. How and why ethanol is denaturated ?
Q27. Why are carbon and its compound used as fuels for most applications ?
Q28. Why is Ethanoic acid named as Glacial Acetic acid ?
Q29. What is Vinegar chemically?
Q30. Define the terms Hetero-atom and a suffix. Explain with examples.
Q31. Explain the nature of covalent bond using the formation of bond in CH3Cl.
Q32. Draw structure of Cyclohexane.
Q33. What do you mean by structural isomers ? Give one example.
Q34. Which is good for health a vegetable oil or Animal fat and why?
Q35. Explain alkane, alkene and alkyne with suitable example.
Q36. Complete the following chemical reactions :
(i) CH3-CH2-OH + O2
(ii) CH3CH2OH
(iii) CH3COOC2H5
(iv) CH3COOH + NaHCO3
(v) Na + C2H5OH
(vi) C2H5OH
Q37. Identify alkanes, alkenes and alkynes in following:
C2H2, C2H4, C3H8, C4H10, C4H8, C5H8
Q38. Write a short note on Micelles.
HBSE Class 10 Science Biology Portion Important Questions 2024
HBSE Class 10 Science Chapter 5 – Life Processes Important Questions 2024
Q1. Describe the excretion in plants. Most Important
Q2. Describe digestion in small intestine.
Q3. Write down the functions of various digestive enzymes to digest the food in alimentary canal.
Q4. Describe the respiratory system of human beings. Most Important
OR
Explain the structure of human respiratory system with the help of well labelled diagram.
Q5. How does phototropism occur in plants?
Q6. Describe the structure of nephron and how is urine produced? Most Important
OR
Describe structure and functioning of nephron. Most Important
Q7. Draw a well labelled diagram of excretory system and explain the process of urine formation in human being.
OR
Explain the structure of human excretory system with the help of a well labelled diagram.
Q8. Explain the process of double circulation with the help of schematic representation of oxygen and carbon dioxide transport in human beings.
Q9. How is the food taken and digested in Amoeba and Paramecium ?
Q10. What is lymph ? How is it transported ? What are its functions ? Most Important
Q11. What is transpiration? How does it take place? What is its role?
Q12. What are the functions of transpiration?
Q13. What is Excretion? Describe the structure of nephron with the help of well labelled diagram.
Q14. How do living things get their food? Write various strategies.
Q15. What is autotrophic nutrition? Explain the process of photosynthesis in plants.
Q16. What is heterotrophic nutrition? Explain the process of nutrition in Amoeba with diagram.
Q17. Describe structure and functioning of human heart. Most Important
Q18. What part of plant transports water? Explain the movement of water and minerals in plants.
Q19. Draw a well labelled diagram of schematic sectional view of Human Heart.
Q20. Differentiate between Artery and Vein.
Q21. Describe various pathways of glucose breakdown in organisms.
Q22. Draw a well labelled diagram of cross-section of leaf. Most Important
Q23. How are the lungs designed in human beings to maximize the area for exchange of gases?
Q24. Draw a well labelled diagram of human digestive system.
Q25. Describe the process of digestion of fat.
Q26. What is difference between lymph and blood?
Q27. What are the differences between aerobic and anaerobic respiration? Most Important
Q28. Write three differences between autotrophic and heterotrophic nutrition.
Q29. How is oxygen and carbon-dioxide transported in human beings?
Q30. What are the necessary conditions for autotrophic nutrition and what are its byproducts ?
Q31. What are the differences between transport of Materials in xylem and phloem?
Q32. The inner lining of small intestine has numerous finger like projections called __________.
Q33. What is photosynthesis? Write various events which take place during this process. Most Important
Q34. Draw a well labelled diagram of stomata. Explain how does the stomatal pore open and close ?
Q35. By which part of plant translocation of food takes place ? Where is the food translocated ?
Q36. Why terrestrial organisms have advantage over aquatic organisms with regard to obtaining oxygen for respiration?
HBSE Class 10 Science Chapter 6 – Control and Coordination Important Questions 2024
Q1. What is geotropism ? Give an example. Most Important
Q2. Draw a well labelled diagram of a neuron. Most Important
Q3. What happens at synapse between two neurons? Most Important
Q4. What is reflex arc ? What is the function of sensory neuron in reflex arc ?
Q5. Draw a well labelled diagram of Reflex arch.
Q6. What is the role of the brain in reflex action ?
Q7. What is the function of receptors in our body ? Write about two types of receptors.
Q8. What is peripheral nervous system?
Q9. What are the various parts of peripheral nervous system? Write their functions.
Q10. Write down the functions of cerebellum and medulla. Most Important
Q11. What is the effect of hormone Adrenalin ?
Q12. What are the functions of Abscisic acid?
Q13. What is called peripheral nervous system? Write its different parts.
Q14. What are the functions of cytokinins?
Q15. Why is the use of iodised salt advisable ? Write the functions of the hormone secreted by thyroid gland.
Q16. What are the functions of Gibberellin?
Q17. What are the functions of forebrain?
Q18. What is the name of the gland secreting adrenaline hormone?
Q19. Iodine is necessary for the synthesis of which hormone?
Q20. Name the hormone responsible for limiting the sugar level in blood in human beings.
Q21. Explain the process of response to touch in sensitive plant.
Q22. How are brain and spinal cord protected in human body?
Q23. Which gland secrete the growth hormone? What happens due to deficiency and excess of this hormone?
Q24. Name the mechanism by which hormone secreted in precise quantity. Give an example.
Q25. Which structure protects spinal cord ?
Q26. What are the various parts of central nervous system? Write their functions.
Q27. What are the limitations to the use of electrical impulses ?
HBSE Class 10 Science Chapter 7 – How do Organisms Reproduce Important Questions 2024
Q1. Which organism cause Kala-azar ?
Q2. What is Germination ?
Q3. Describe the process of budding in Hydra. Most Important
Q4. Write about spore formation in Rhizopus.
Q5. Draw a well labelled diagram of Longitudinal section of a flower. Most Important
Q6. Why are the testes located outside the abdominal cavity in scrotum ?
Q7. Name various glands associated with male reproductive system & write their role.
Q8. Draw a well labelled diagram of human female reproductive system.
OR
Explain human female reproductive system with diagram.
Q9. What happens when the egg is not fertilized?
OR
Why does menstruation occur?
Q10. Write about the process of fission in various organisms.
Q11. What is Pollination ? Write about self and cross pollination.
Q12. In which organism fragmentation occur ? Write about its process.
Q13. Discuss the structure of female reproductive part of a flower.
Q14. Draw a well labelled diagram of seed germination.
Q15. What is the role of the seminal vesicles and the prostate gland?
Q16. How is the process of pollination different from fertilization? Most Important
Q17. Describe three different methods of contraception.
Q18. What is the name of the process of formation of new individual in Amoeba?
Q19. What is function of testis in man?
Q20. What are unisexual and bisexual flowers? Give examples.
Q21. What is Regeneration ?
Q22. What is the function of ovary in human beings?
Q23. What is vegetative propagation? How is it useful?
Q24. Which organism divides into many daughter cells through multiple fission?
Q25. Write those changes of puberty that are common to girls and boys.
Q26. Growth of facial hair and deepening of voice are symptoms of puberty in boys. Which hormone is responsible for it?
Q27. In a flower, pollen tube enters through pistil into ovule. Name that part of pistil.
Q28. What is difference between binary fission and multiple fission ? Explain with example.
Q29. What are different contraceptive methods? How are they important for reproductive health? Most Important
Q30. What are the changes seen in girls and boys at time of puberty?
Q31. Describe the importance of variation.
HBSE Class 10 Science Chapter 8 – Heredity Important Questions 2024
Q1. How does the mechanism of hereditary work?
Q2. How do Mendel’s experiment show that traits are inherited independently? Most Important
Q3. How do Mendel’s experiments show that traits may be dominant or recessive ?
Q4. How is the sex of the child determined in human beings? Most Important
Q5. What is meant by characteristics?
Q6. Gregor Mendel belonged to which country?
Q7. Mendel crossed a tall pea plant (TT) and a short plant (tt) and produced progeny (Fi) from them. All plants were _________.
Q8. As per Mendel’s Law, describe the independent assortment of two separate traits (rounded and green seeds) with ( wrinkled and yellow seeds) along with diagram.
Q9. Rounded seeds (dominant trait) were crossed with wrinkled seeds (recessive trait).
(i) What type of seeds will be produced in F1 generation ?
(ii) By self-pollination in F₁ seeds, what percentage of seeds will be rounded shape in F2 generation ?
Explain with diagram.
Q10. Which sex chromosomes is present in females ?
HBSE Class 10 Science Chapter 13 – Our Environment Important Questions 2024
Q1. Define ecosystem.
Q2. What are biodegradable substances?
Q3. What are the various biotic and abiotic components of Ecosystem?
Q4. “Flow of energy is unidirectional in ecosystem”, explain.
Q5. In how many directions the energy flows in any ecosystem ?
Q6. What percentage of solar energy captured by green plants is converted into food energy in a terrestrial ecosystem?
Q7. Write full form of UNEP. Most Important
Q8. The autotrophs capture the energy present in sunlight and convert it into ________ energy.
Q9. The higher energy UV radiations split apart some molecular oxygen into _________ atom.
Q10. Which layer shields the surface of earth from ultraviolet radiations form Sun?
Q11. Name the solar radiation from which the surface of earth is shielded by ozone layer.
Q12. Which synthetic chemical is responsible for depletion of ozone layer?
Q13. What are the advantages and disadvantages of ozone ?
Q14. Which organisms act as decomposer ?
Q15. Write full form of CFCs.
Q16. What is the role of decomposers?
Q17. What are trophic levels?
HBSE Class 10 Science Physics Portion Important Questions 2024
HBSE Class 10 Science Chapter 9 – Light : Reflection and Refraction Important Questions 2024
This Table is Most important for this chapter. Must revise this.
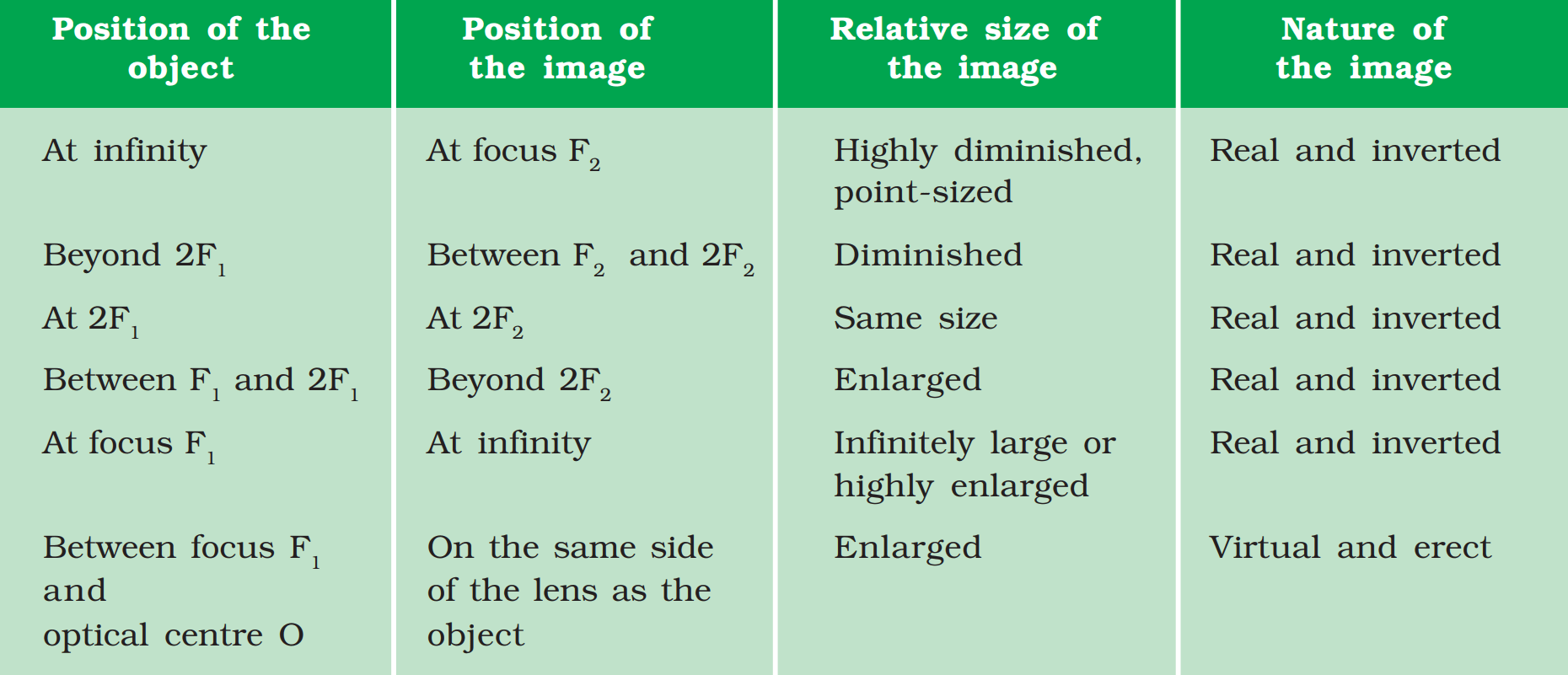
1. Nature, position and relative size of the image formed by a convex lens for various positions of the object
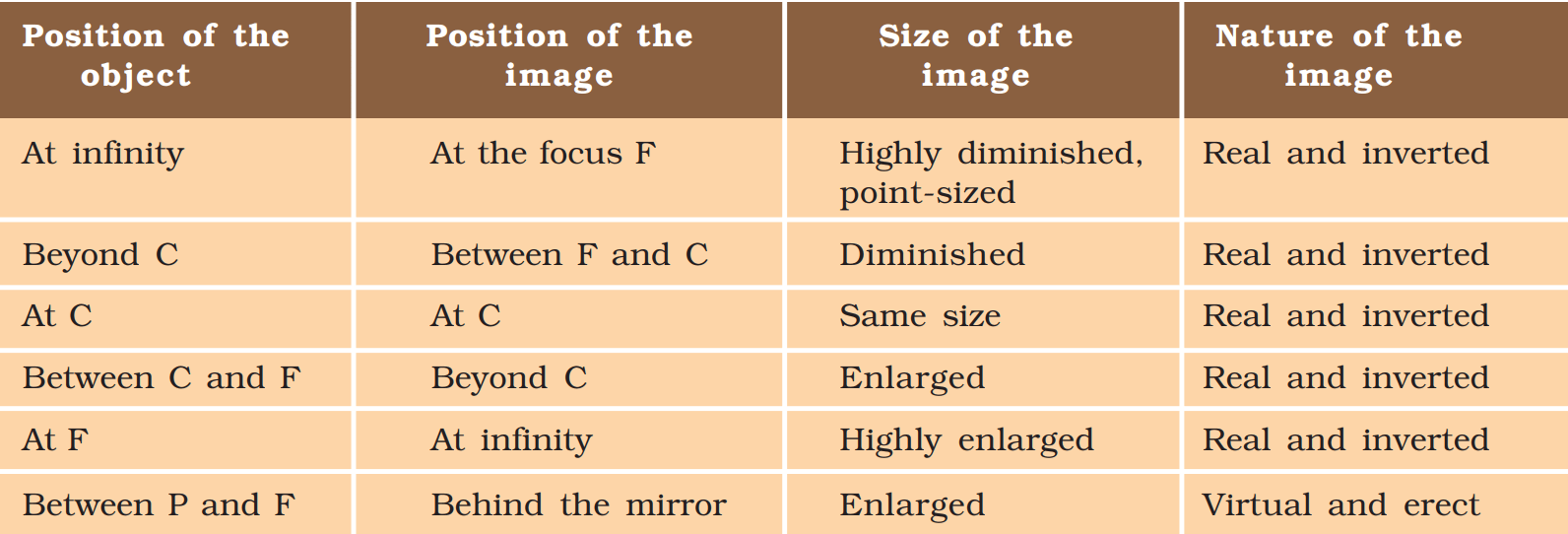
2. Image formation by a concave mirror for different positions of the object
Q1. Using a ray diagram, explain the position, relative size and nature of image of an object placed between the F1 and 2F1 of a convex lens.
Q2. Using a ray diagram, explain the position, relative size and nature of image of an object placed beyond 2F1 of a convex lens.
Q3. Using ray diagram, explain position, size and nature of image of an object placed beyond centre of curvature C of a concave mirror.
Q4. Using ray diagram, explain position, size and nature of image of an object placed between focus F and centre of curvature C of a concave mirror.
Q5. What is power of a Lens ? Define one Dioptre (1D) power of a lens. Most Important
Q6. Why do we prefer a convex mirror as a rear view mirror in vehicles?
Q7. An object is placed at the centre of curvature (c) of a concave mirror. Draw the ray diagram to depict the position, size and the nature of image formed. Most Important
Q8. An object is placed at a position in between the main focus (F1) and the optical centre (O) of a convex lens. Draw a ray diagram showing the position, size and nature of the image formed. Most Important
Q9. Draw ray diagram and write nature of image formed if an object is placed at:
a) Between F1 and 2F1 of a concave lens.
b) Between F1 and 2F1 of a convex lens.
Q10. Find the focal length of a lens of power -2.0 D. What type of lens is this? Most Important
Q11. Find the focal length of a lens of power +2.0 D. What type of lens in this ? Most Important
Q12. Light enters from air to glass having refractive index 1.50. What is the speed of light in glass? The speed of light in vacuum is 3 × 108 ms-1.
Q13. An object 5.0 cm in length is placed at a distance of 20 cm in front of a convex mirror of radius of curvature 30 cm. Find the position of the image, its nature and size.
Q14. A concave lens has focal length of 15 cm. At what distance should the object from the lens be placed so that it forms an image at 10 cm from the lens? Also, find the magnification produced by the lens.
Q15. Find the focal length of a convex mirror whose radius of curvature is 32 cm. Most Important
Q16. lf an object is placed on the infinity of a concave lens, then draw a ray diagram and explain the position size and nature of its image.
Q17. Write the laws of reflection of light.
Q18. Write the laws of refraction of of light. Most Important
Q19. State the types of mirror used for:
(i) headlights in car
(ii) rear view mirror in any vehicle
(iii) solar furnace
Give reason to justify your answer in each case.
Q20. Write the lens formula.
Q21. Write the mirror formula.
HBSE Class 10 Science Chapter 10 – The Human Eye and The Colourful World Important Questions 2024
Q1. What type/nature of image is formed by the eye lens at retina ?
Q2. What is the function of iris ?
Q3. Which phenomenon of light is responsible for twinkling of stars?
Q4. Explain why the planets do not twinkle. Most Important
Q5. Why do stars twinkle?
Q6. The prism split the incident white light into a band of colours. Write these colours name in sequence.
OR
Write the name of colours in sequence found after splitting of white light passing through a glass prism.
Q7. Why does the sky appear dark instead of blue to an astronaut ?
HBSE Class 10 Science Chapter 11 – Electricity Important Questions 2024
Q1. The potential difference between the terminals of an electric heater is 80 V, when it draws a current of 5 A from the source. What current will the heater draw, if the potential difference is increased to 160 V?
Q2. An electric motor takes 4 A from a 220 V line. Find the power of the motor and energy consumed in 3 hours.
Q3. Why are the coils of electric irons and toasters are made of an alloy rather than a pure metal ? Most Important
Q4. 150 Joule of heat is produced each second in a 6Ω resistance. Find the potential difference across the resistor.
Q5. A 6 Ω resistance emits heat energy at the rate of 125 J / s. Find the potential difference across the resistor.
Q6. How can three resistors of resistances 2Ω, 3Ω and 6Ω be connected to give a total resistance of 1Ω ? Most Important
Q7. A lamp of resistance 20 Ω and a conductor of resistance 8 Ω are connected with a battery of 8 V in series. Calculate the :
(a) total resistance in the circuit
(b) current flowing through the circuit
Q8. An electric refrigerator of power 400 W is allowed to run 10 hrs. per day. What is the cost of energy to operate it for 30 days at Rs. 4.00 per kWh? Most Important
Q9. On what factors does the resistance of a conductor depend? Most Important
Q10. What do you mean by earthing? Why should electrical appliances be earthed ? Most Important
Q11. Why is tungsten used almost exclusively for filament of electric lamps?
Q12. An electric current of 0.5 A flows through the filament of an electric bulb for 5 min. What will be the electric charge flowing through that wire ?
Q13. An electric bulb is connected to a 220 V generator. The current is 0.50 A. What is the power of the bulb?
Q14. When a 12 V battery is connected across an unknown resistor there is a current of 2.5mA in the circuit. Find the value of resistance of the resistor.
HBSE Class 10 Science Chapter 12 – Magnetic Effect of Electric Current Important Questions 2024
Q1. Explain Right hand thumb rule. Most Important
Q2. Briefly explain the Fleming’s left hand rule.
Q3. Write two properties of magnetic lines of force.
Q4. Describe the magnetic field due to current in a solenoid.
Q5. What is a Solenoid? Draw the magnetic lines of force around a current carrying solenoid. Also throw some light on the use of solenoid. Most Important
Q6. Describe the magnetic field due to current through a circular loop.
Q7. Draw the magnetic lines of force around a bar magnet. Most Important
Q8. What do you mean by electromagnetic induction? Explain the use of Fleming’s right hand rule in finding the direction of current induced in the conductor. Most Important
Q9. Explain the force on a current carrying conductor in a magnetic field. Write about the rule to find direction of this force. What are the devices in which current carrying conductors and magnetic fields are used?
Q10. Explain the magnetic field due to current through a straight conductor. Describe the rule to find the direction of such a magnetic field.
Q11. What are the two safety measures commonly used in electric circuits and appliances? Explain their working.