NCERT Class 9 Science Chapter 1 Matter in Our Surroundings Exercise Question Answer Solution For School Students of Class 9th. We also Provides Notes and Important Questions for Class 9 Science. NCERT Class 9th Science book is applied in mostly boards like CBSE, HBSE, RBSE, Up Board, MP Board and also some other state boards.
Also Read:- Class 9 Science NCERT Solution
NCERT Class 9 Science Chapter 1 Matter in Our Surroundings Question and Answer for CBSE, HBSE and Other Boards Solution.
Matter in Our Surroundings Class 9 Science Question Answer
Q1. Convert the following temperatures to the Celsius scale.
(a) 293 K
(b) 470 K
Ans. As we know that 0°C = 273K
So,
(a) 293 K = (293 – 273)°C = 20°C .
(b) 470 K = (479 – 273)°C = 206°C .
Q2. Convert the following temperatures to the kelvin scale.
(a) 25°C
(b) 373°C
Ans. As we know that 0°C = 273K
Therefore,
(a) 25°C = (273 + 25)K = 298K
(b) 373°C = (273 + 373)K = 646K
Q3. Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
Ans. Naphthalene balls disappear with time without leaving any solid because at room temperature Naphthalene undergoes sublimation reaction in which it directly converted into gas without change into liquid.
(b) We can get the smell of perfume sitting several metres away.
Ans. We can get the smell of perfume sitting several metres away due to high speed of particles and large space between them. Perfumes comprise of flavoured substances that are volatile which scatters quickly in air, becoming less concentrated over a distance.
Q4. Arrange the following substances in increasing order of forces of attraction between the particles – water, sugar, oxygen.
Ans. Increasing of order of forces of attraction between the particles follows the order gas<liquid<solid.
Therefore, the correct increasing order of forces of attraction is oxygen ( gas ) < water ( liquid) < sugar (solid).
Q5. What is the physical state of water at –
(a) 25°C
(b) 0°C
(c) 100°C
Ans. The physical state of water at given temperatures is as :
At 25°C, water is liquid.
At 0°C, water is at its freezing point, so it may be in solid state or in liquid state.
At 100°C, water is at its boiling point so it may be at liquid state or in gaseous(either vapor ).
Q6. Give two reasons to justify –
(a) water at room temperature is a liquid.
Ans. Water at room temperature is a liquid due to its freezing point is 0°C and its boiling point is 100°C.
Reasons –
1. At this temperature shape of water is not fixed.
2. Also water has fixed volume at room temperature.
(b) an iron almirah is a solid at room temperature.
Ans. An iron almirah is a solid at room temperature because its melting and boiling points are much higher than room temperature.
Reasons –
1. Metals have high melting and boiling point.
2. Metals have high intermolecular force of attraction.
Q7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Ans. Ice at 273K is more effective in cooling than water because ice has stored more latent heat or heat energy as compared to water. When fusion took place ice will absorb more heat and provide more cooling as compared to water.
Q8. What produces more sever burns, boiling water or steam?
Ans. Steam provides more sever burns as compare to boiling water. This is due to the reason that steam contains more heat energy as compared to boiling water during the process of vaporization.
Q9. Name A, B, C, D, E and F in the following diagram showing change in its state.
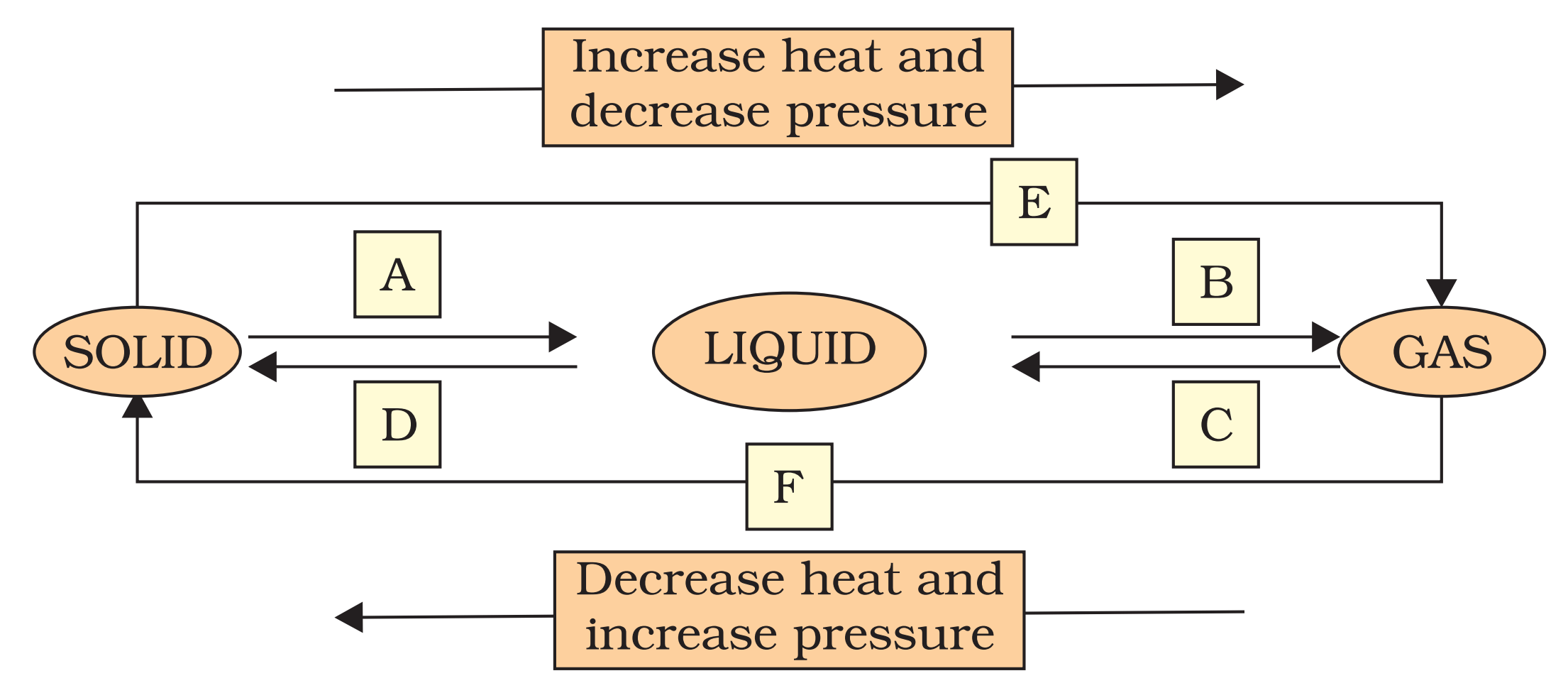
Ans.
A —> Liquefication/melting/fusion
B —> Vapourisation/evaporation
C —> Condensation
D —> Solidification
E —> Sublimation
F —> Sublimation